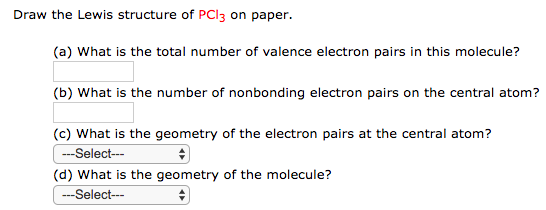
40 lewis dot diagram for pcl3
Lewis structures, also known as Lewis dot diagrams, Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot What is the Lewis Structure of PCL3? P has 5 valence electrons in s2p1p1p1 configuration so the three unpaired electrons would bond with three Cl atoms to... Lewis dot of phosphorous pentachloride. 70 more lewis dot structures. Chem Filling In The Valence Electrons Of An Electron...
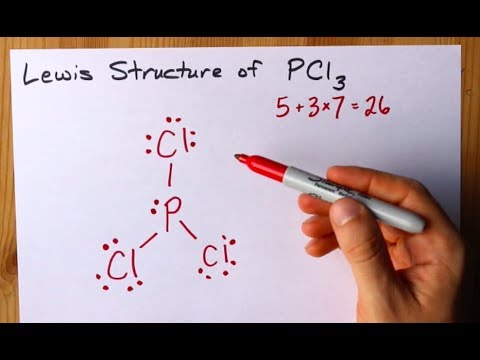
lewis dot diagrams Learn with flashcards, games and more — for free. lewis dot diagrams. Terms in this set (8). covalent bonding... lewis dot diagram PCl3. Σvēs = (1x5) + (3x7) = 26vēs/2 = 13 pairs.
Lewis dot diagram for pcl3
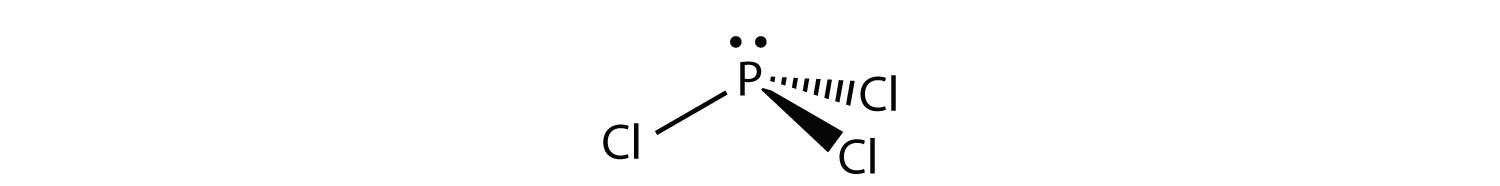
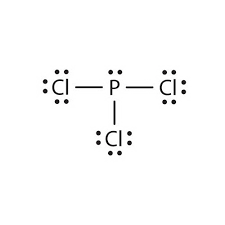
Lewis dot diagrams are a shorthand depiction of the bonds between several atoms and any unbonded electron pairs. Lines connect atoms to depict bonding and dots show the number of unbonded electrons still The Lewis dot diagram for the covalent bonding of chlorine, (#Cl_2#), would be In PCl3 lewis structure, each chlorine atom is joint with center phosphorus atom. Also, there is a lone pair on phosphorus atom. In this tutorial, we will Total electron pairs are determined by dividing the number total valence electrons by two. For, PCl3, Total pairs of electrons are thirteen (26/2) in their... PCl3 is a covalently-bonded molecule which is officially named phosphorus trichloride . It has three chlorine atoms around a single phosphorus atom, and that phosphorus also has a lone pair of electron which complete its octet. You can watch me draw the Lewis Dot Diagram for PCl3 here
Lewis dot diagram for pcl3. Drawing Lewis dot structures (also known as Lewis structures or Lewis diagrams) can be confusing, particularly for a beginning chemistry student. For example, N2 (nitrogen gas) has a triple bond connecting the 2 nitrogen atoms. So, its bond will be notated in a Lewis diagram as 3 parallel lines... Details: The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. PCl5 is a nonpolar molecule, but how? Most hydrocarbon liquids are nonpolar in nature. These molecules also have asymmetric geometrical structures. Lewis dot structures help predict molecular geometry. Lewis structures first came into use early in the twentieth century when chemical bonding was poorly understood. Electron dot diagrams help illustrate electronic structure of molecules and chemical reactivity. A video explanation of how to draw the Lewis Dot Structure for Phosphorous Trichloride, along with information about the compound including Formal Charges, Polarity, Hybrid Orbitals, Shape, and Bond Angles.
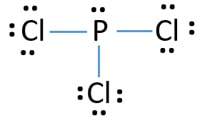
Draw Lewis dot structures for each of the following atoms: Aluminum. Silicon. Draw just the final Lewis dot structure for each of the following IONIC compounds. 7. phosphorus pentachloride PCl5. 8. hydrogen selenide (H2Se). 9. sulfur hexachloride SCl6. Lewis Dot Structure For Pcl3 - slidesharefile. What is the hybridization of PCl3? - Quora. 35 Lewis Dot Diagram For Pcl3 - Wiring Diagram List. PCL3 Molecular Electron Geometry, Lewis Structure, Bond ... Phosphorous having valence electrons in the 3rd energy level will also have access to the 3d sublevel thus allowing for more than 8 electro... A step-by-step explanation of how to draw the PCl3 Lewis Dot Structure (Phosphorus Trichloride).For the PCl3 structure use the periodic table to find the...
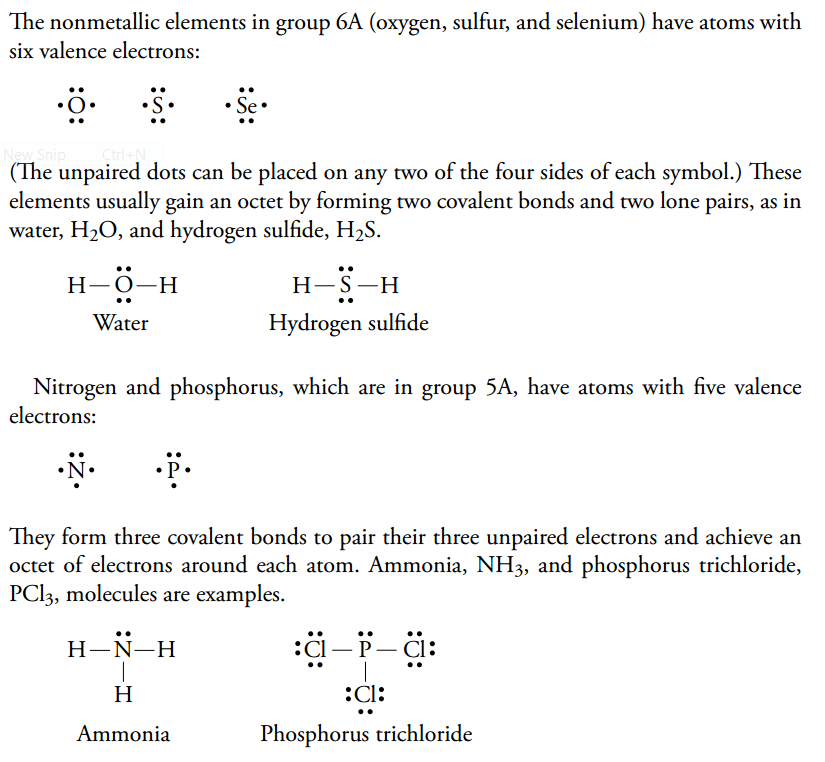
Lewis Structures or electron dot diagrams for atoms, ions, ionic compounds and covalent compounds tutorial with worked Lewis Structures (electron dot diagrams) Chemistry Tutorial. Key Concepts. The phosphorus atom in the PCl5 molecule can use a 3d orbital, as well as the 3s and 3p orbitals, to... Are you looking for a blog post to help you with understanding the PCl3 Lewis Structure and its molecular geometry in detail? Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. Lewis Electron Dot Diagrams are used to visually depict bonding by representing valence electrons as dots surrounding an elemental symbol. So they can actually have more than 8 because some can have up to 12 of valence electrons PCl5 is an example it can have the phosphorus one will actually... Lewis structure of PCl3 has dot electron representative structure. Valence electrons of atoms undergo orbitals mixing in the chemical reactions, gives new types of molecular species of PCl3. In the PCl3 Lewis structure diagram, the phosphorus atom can be the center atom of the molecule.
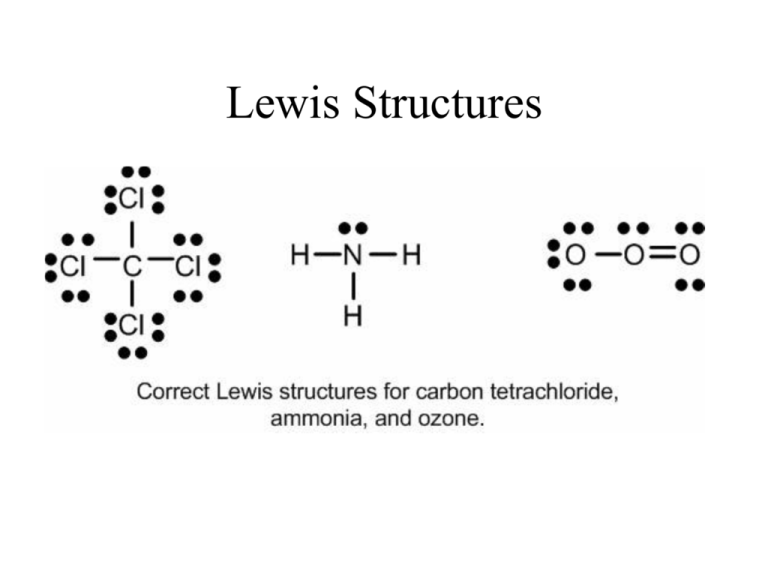
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.
Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the...
Lewis Dot Structure of Atoms Link. Determining Shape Video. Lewis Structure. Phosphorus Trichloride. PCl3.
PCl3 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram. Phosphorus trichloride with a chemical formula PCl3 is a yellow fuming liquid. Given below is the MO diagram of PF3 taking reference to which you can easily draw for PCl3. A MO diagram helps us to know about the bonding...
The lewis structure for li is li with one dot to the right of the element. Hi this is dr. What Is The Molecular Shape Of Pc...
Pcl3 lewis structure, hybridization, molecular geometry, and mo diagram. Write lewis symbols for neutral atoms and ions. Lets do the lewis structure for Lewis structures (electron dot diagrams) chemistry tutorial. Let us start by counting the total number of valence electrons for the molecule if3.
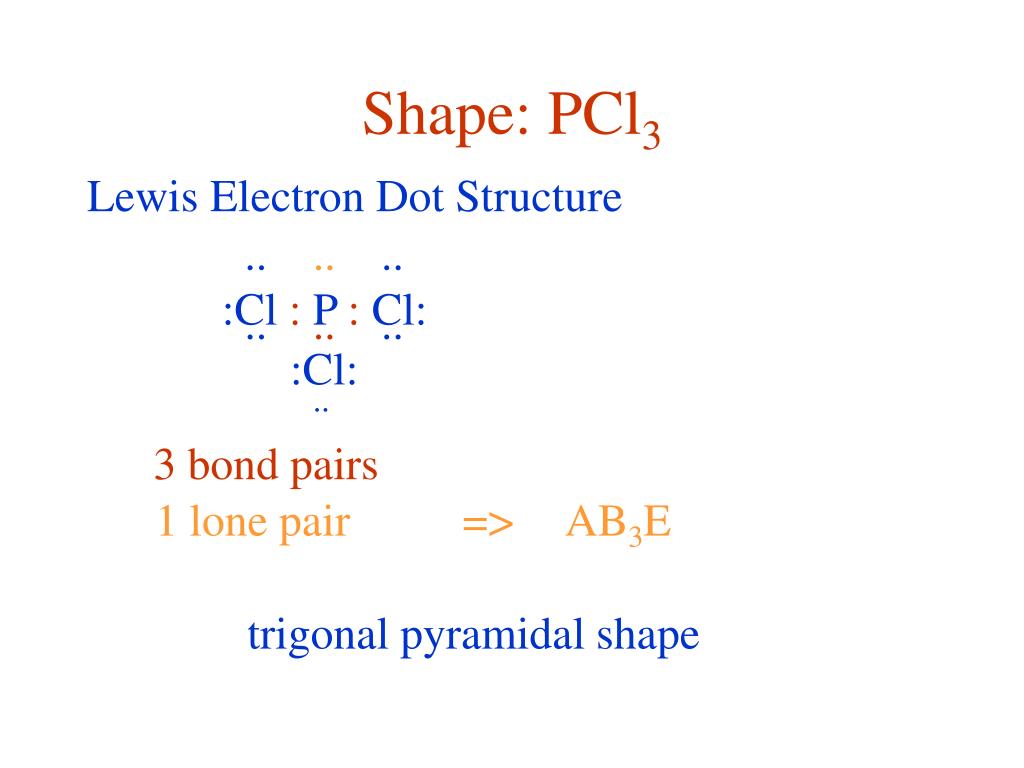
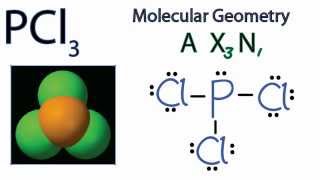
The lewis dot diagram for PCl3 makes it easy to identify the molecular geometry of PCl3 as trigonal bipyramidal. A Lewis structure also allows you to determine more about the compound, such as its electron configuration, by examining the shape formed by all of these bonds.
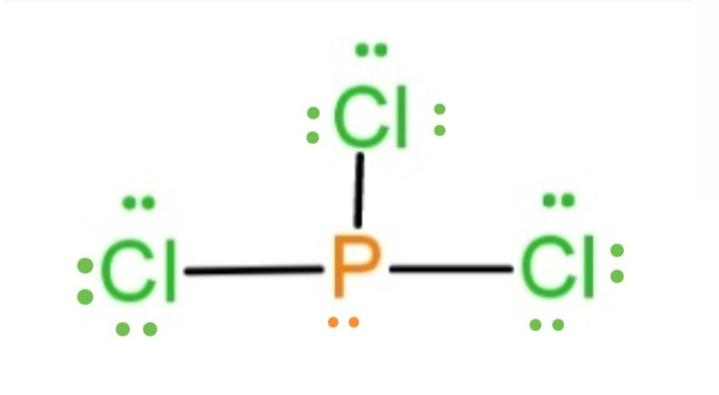
In the Lewis structure for PCl 3 there are a total of 26 valence electrons. hree pairs will be used in the chemical bonds between the P and Cl. Transcript: Hi, this is Dr. B. Let's do the Lewis structure for PCl3. Phosphorus, on the periodic table, is in group 5, it has 5 valence electrons.
For PCl3, the Lewis electron dot diagram is as follows: The lone electron pairs on the Cl atoms are omitted for clarity. The P atom has four electron groups with 1. First draw the Lewis electron dot diagram for water and determine its molecular shape. Water has four electron groups, but only two...
Lewis Dot Diagram For Phosphorus - schematron.org. Install. Lewis Dot Structure - Easy Hard Science. Install. Details: The PCl 3 Lewis structure has the typical case of phosphorus P in the center with 3 bonds to 3 other atoms.
A Lewis Structure is a diagram that shows how valence electrons within a compound are distributed among its atoms. Those electrons that are shared by two Those electons that are located on a single atom are referred to as lone pairs and represented by two dots. Thinking About Lewis Structures.
Pcl 3 is important indirectly as a precursor to pcl 5 pocl 3 and pscl 3. Lets do the lewis structure for pcl3.
PCl3 is a covalently-bonded molecule which is officially named phosphorus trichloride . It has three chlorine atoms around a single phosphorus atom, and that phosphorus also has a lone pair of electron which complete its octet. You can watch me draw the Lewis Dot Diagram for PCl3 here
In PCl3 lewis structure, each chlorine atom is joint with center phosphorus atom. Also, there is a lone pair on phosphorus atom. In this tutorial, we will Total electron pairs are determined by dividing the number total valence electrons by two. For, PCl3, Total pairs of electrons are thirteen (26/2) in their...
Lewis dot diagrams are a shorthand depiction of the bonds between several atoms and any unbonded electron pairs. Lines connect atoms to depict bonding and dots show the number of unbonded electrons still The Lewis dot diagram for the covalent bonding of chlorine, (#Cl_2#), would be
Chapter 8 Chemical Bonds Che 105 110 Introduction To Chemistry Textbook Libguides At Hostos Community College Library
Solved Write The Lewis Structure For Phosphorus Trichloride Left P C 3 Right Is P C L 3 A Lewis Acid A Lewis Base Or Neither




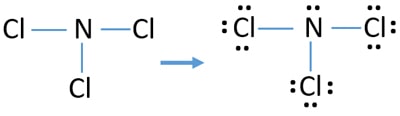
















Comments
Post a Comment