41 co orbital diagram
What is the orbital diagram of CO 27? In this case of Cobalt there are 27 electrons which are present in 4 orbits and their distribution on the orbit that is electronic configuration can be written as: 1s22s22p63s23p63d74s2. Draw MO diagram of CO and calculate its bond order. chemical bonding; class-11; Share It On Facebook Twitter Email. 1 Answer +1 vote . answered Dec 17, 2020 by Maisa (45.7k points) selected Dec 18, 2020 by Panna01 . Best answer. 1. Electronic configuration of C atom: 1s 2 2s 2 2p 2. ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...

Co orbital diagram
Carbon Monoxide Molecular Orbital Diagram Explanation. generic s-p valence MO diagram for carbon monoxide CO chain one can reasonably explain, that the HOMO of carbon monoxide must be of. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining MO diagrams can explain why some molecules exist and others do not. Co orbital diagram. Cobalt has a total of 27 electrons which are contained in 1s 2s 2p 3s 3p 4s and 3d sub. Controls click on the co molecular orbitals in the energy level diagram to display the shapes of the orbitals. So you have the carbon two s orbital and you have the carbon two p orbitals. Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of ...
Co orbital diagram. The molecular orbital diagram of carbon monoxide, CO, is show below. Which overlap is strongest? During the axial overlap of p-p orbitals, the electron density increases around the axis, so the bond formed is the strongest. Therefore, the strongest bond formed is when p-p orbital overlap occurs. Final answer: The correct answer is Option B- 2p ... Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. 5.4.2: Carbon Dioxide. Construct SALCs and the molecular orbital diagram for CO 2. Step 1. Find the point group of the molecule and assign Cartesian coordinates so that z is the principle axis. Step 2. Identify and count the pendant atoms' valence orbitals. Step 3. Generate the Γ 's. Step 4.
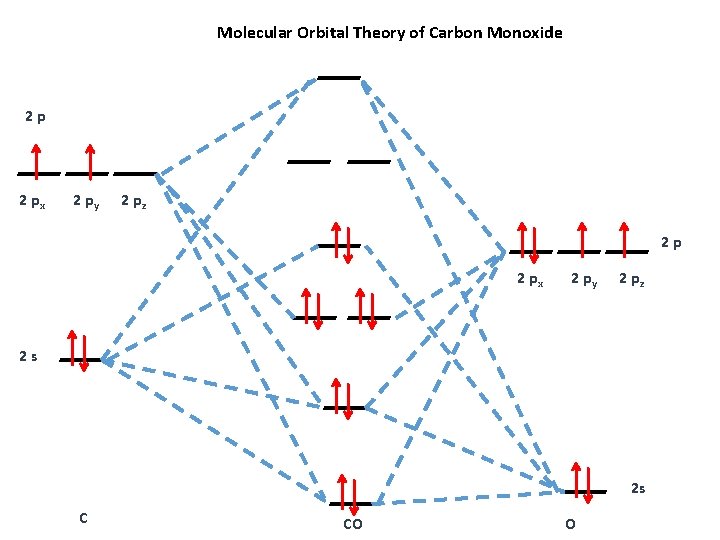
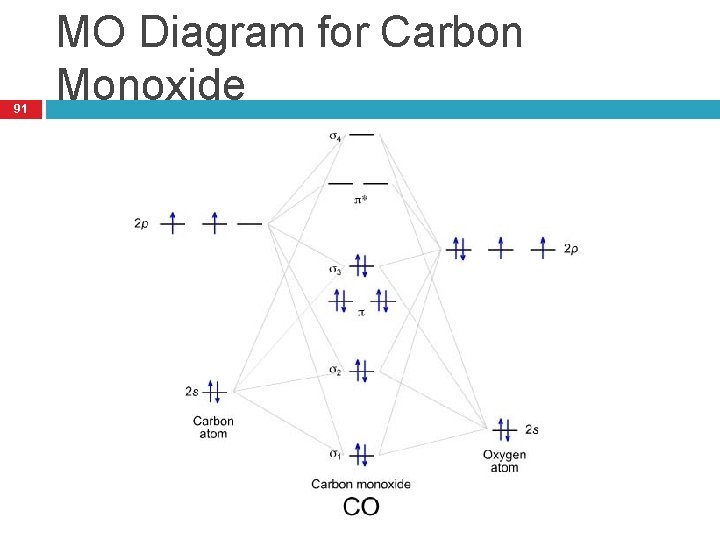
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine Molecular Orbital Diagram of CO. TAGS; Molecular Orbital Diagram; Previous article Wohl-Ziegler Bromination. Next article Molecular Orbital Diagram of NO. All About Chemistry. https://allaboutchemistry.net. Hello Reader! Thanking for reading this post, If you find it to be informative, pls share it and visit our website. Answer (1 of 10): bond order = 3 Explanation = (1) no of electron in C = 6 (2) no of electron in O = 8 So, total no of electron = 14, so bond order = 3 Trick No. of electron = Bond order 12 = 2 13 = 2.5 14 = 3 15 = 2.5 16 = 2 17 =1.5 18 = 1 The valence molecular orbitals in both atoms are the 2 s and 2 p orbitals. The molecular orbital diagram for carbon monoxide (Figure 5.3.1. 1) is constructed similarly to how you would construct dicarbon or dioxygen, except that the oxygen orbitals have a lower potential energy than analogous carbon orbitals. The labeling of molecular orbitals ...
Co-orbital configuration. In astronomy, a co-orbital configuration is a configuration of two or more astronomical objects (such as asteroids, moons, or planets) orbiting at the same, or very similar, distance from their primary, i.e. they are in a 1:1 mean-motion resonance. (or 1:−1 if orbiting in opposite directions ). The electron configuration of "Co"^(3+) is ["Ar"] 4s 3d^5. "Co" is in Period 4 of the Periodic Table, and "Ar" is the preceding noble gas. Cobalt is also in Group 9, so it must have 9 valence electrons. The valence shell configuration is therefore 4s^2 3d^7, and the core notation is bb"Co": ["Ar"] 4s^2 3d^7 When a transition metal forms an ion, the s electrons are removed before the d electrons. Mo Diagram Of Co.In this video we are discuss about MO Diagram and Characteristics of CO Molecule MO diagrams of Heteronuclear Diatomic. A molecular orbital diagram or MO diagram for short is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals molecular orbital method (LCAO method ... Here we have a molecular orbital diagram for the CO molecule. So again, it's drawn in the familiar pattern. You have the, here on this side you would have the energy, so the energy is going up there. So you have the carbon two S orbital, and you have the carbon two P orbitals.
Molecular Orbital Description of the CO Ligand The CO LUMO orbitals are antibonding of * symmetry. These are empty orbitalsand canaccept electron density from a metal centre via ‐ backbonding with the metal d(xy), d(xz) and d(yz), orbitals The CO HOMO orbital is a bonding orbital of symmetry with significant electron density on the carbon.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
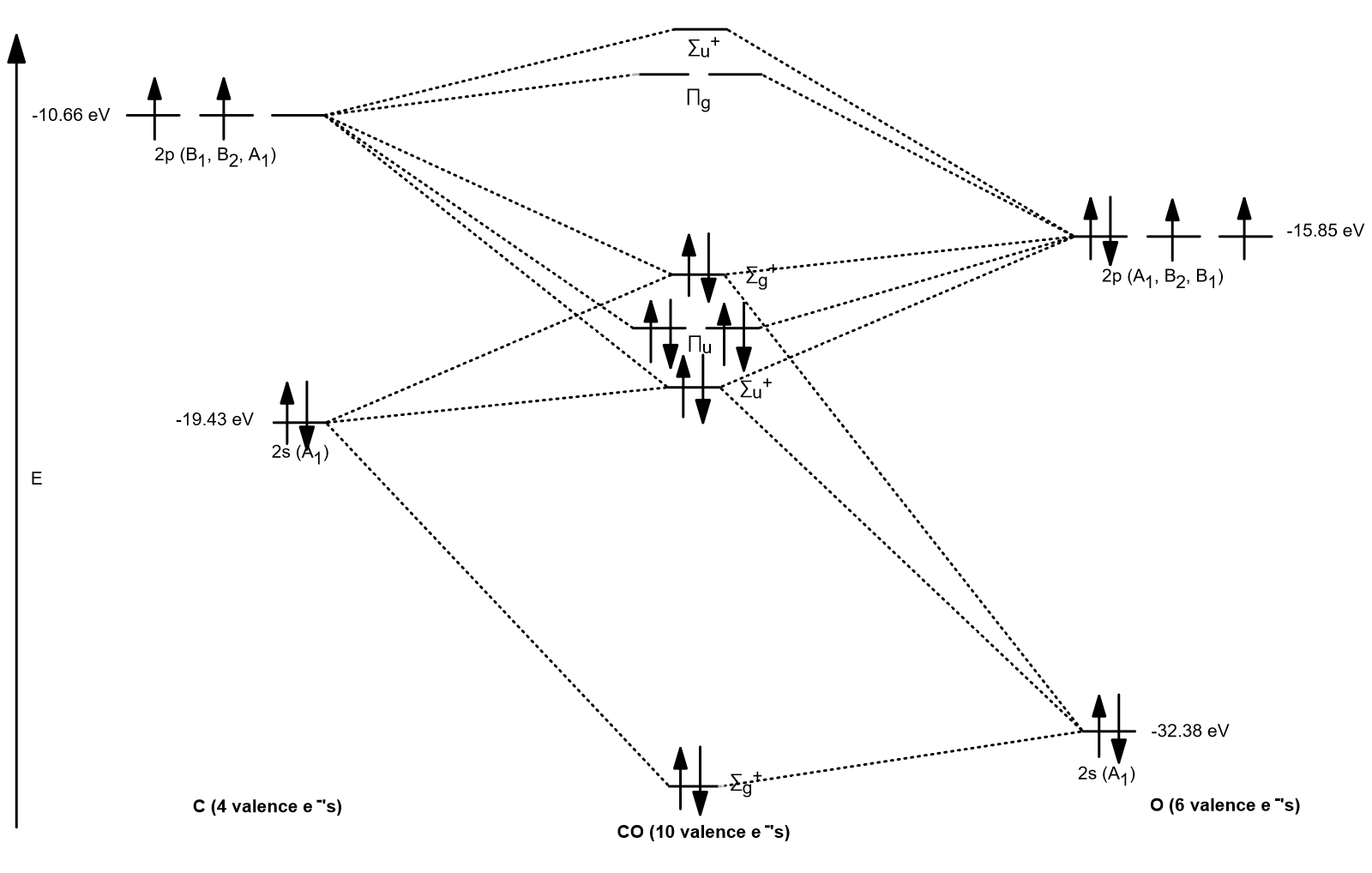
Molecular Orbital Diagram of Carbon Monoxide (CO) The above image shows energy levels for the molecular orbitals of the carbon monoxide (CO) The molecular orbital diagram is a diagrammatic representation of showing how chemical bonding is taking place within a molecule.
Watch the video solution for the question: Draw the orbital diagram for ion Co 2+.. . Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the.Oct 05, · Also Cobalt orbital diagram example problem.
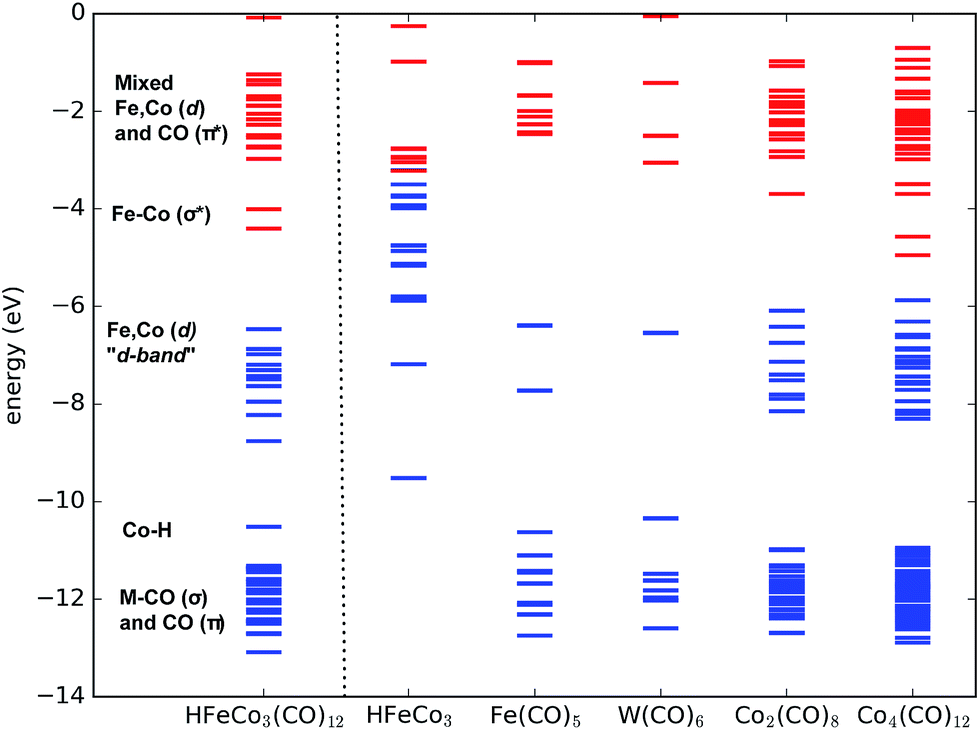
Formation And Decay Of Negative Ion States Up To 11 Ev Above The Ionization Energy Of The Nanofabrication Precursor Hfeco 3 Co 12 Chemical Science Rsc Publishing Doi 10 1039 C7sc01927k
Molecular orbital diagram of CO and charge localisation. Ask Question Asked 1 year, 5 months ago. Active 1 year, 5 months ago. Viewed 110 times 4 1 $\begingroup$ MO diagram of CO. My question concerns the interpretation of the Molecular Orbital of CO. I think I find it clear how you build it but I have some concerns about how you rationalize it.
Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the. Watch the video solution for the question: Draw the orbital diagram for ion Co 2+.. . can be accommodated in the metal d orbitals. • d0 ions •d7 ions - Fe1+, Ru1+, Co2 ...
Orbital Diagrams: Orbital diagrams can be used to represent the electronic configuration of an atom. The orbital diagram is a pictorial representation of the electrons in the atom.
Molecular orbitals diagrams of [Co (NH3)6]3+. 1. M. O. diagram for [Co (NH3)6]3+ Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa. 2. t* 1u a1g t2g, eg a1g, t1u, eg a1g t1u a* 1g e* g eg t1u Δo t2g Metal (Ti3+)orbitals Co3+→ [Ar] 3d6, 4s0 6e- Ligand group (NH3) orbitals 6 x 2 = 12 e- σ [Co (NH3)6]3 ...
Orbital-orbital Interactions and Symmetry Adapted Linear Combinations; ... Molecular orbitals in Carbon Monoxide. CONTROLS > Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules.
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
Orbital Diagram For Chromium Orbital Diagrams Electron Configurations For Atoms And Ions. Orbital Diagram For Chromium Figure 3 From Circular Dichroism Of Trigonal Dihedral Chromiumiii. Orbital Diagram For Chromium A Series Of C3 Symmetric Heterobimetallic Crm M Fe Co And Cu. Orbital Diagram For Chromium Chromium Wikiwand

Co Nh3 6 3 Ion 4 Construct The Mo Diagram Label All Atomic Group And Molecular Orbitals With Homeworklib
Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of ...
Co orbital diagram. Cobalt has a total of 27 electrons which are contained in 1s 2s 2p 3s 3p 4s and 3d sub. Controls click on the co molecular orbitals in the energy level diagram to display the shapes of the orbitals. So you have the carbon two s orbital and you have the carbon two p orbitals.
Carbon Monoxide Molecular Orbital Diagram Explanation. generic s-p valence MO diagram for carbon monoxide CO chain one can reasonably explain, that the HOMO of carbon monoxide must be of. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining MO diagrams can explain why some molecules exist and others do not.
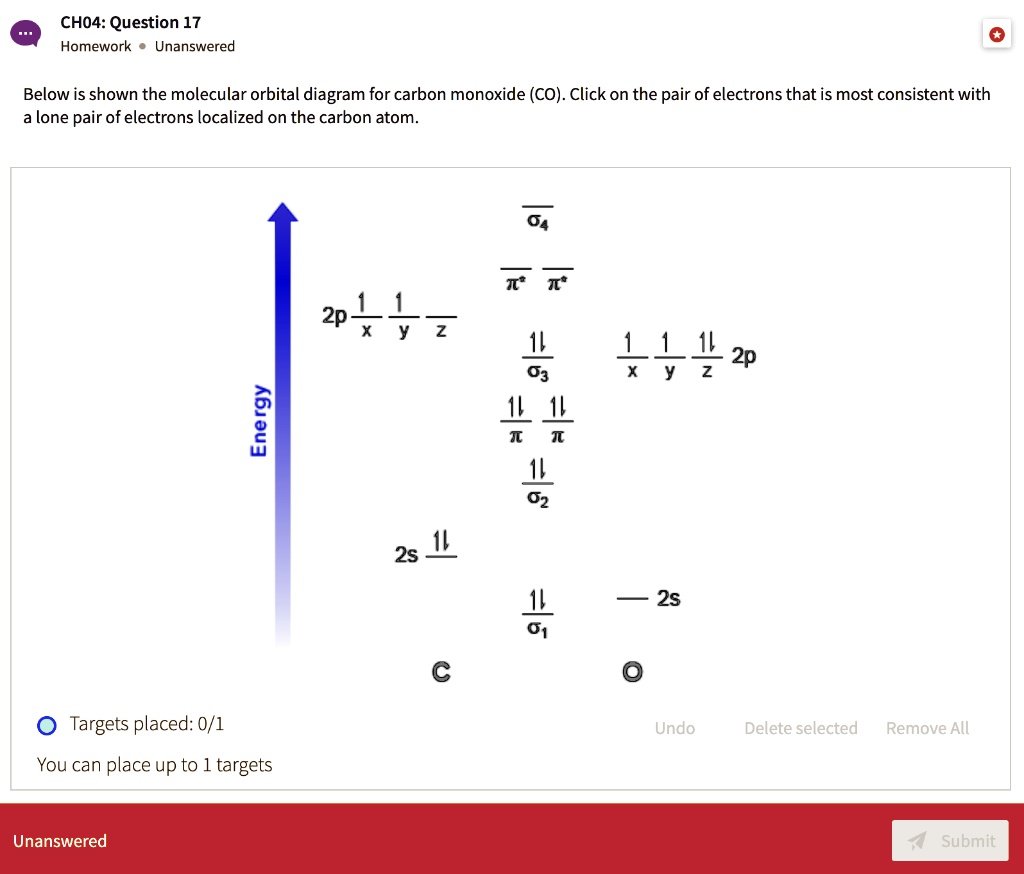
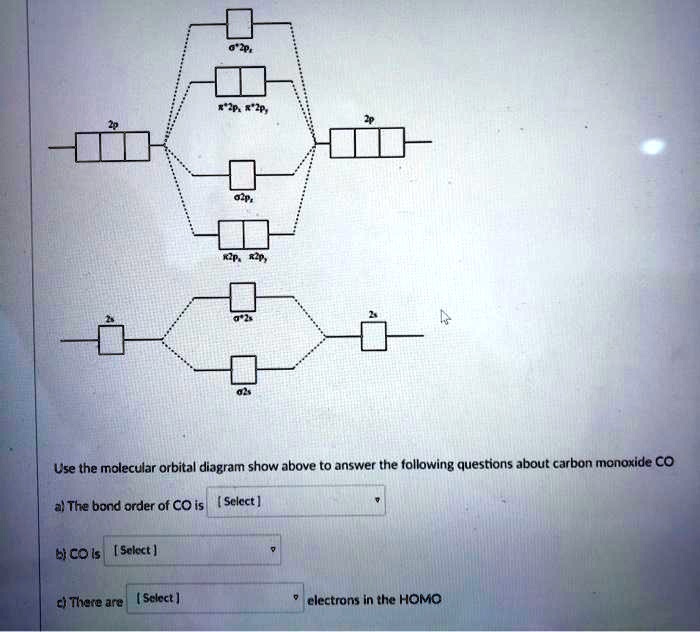
Solved Choa Question 17 Homework Unanswered Below Is Shown The Molecular Orbital Diagram For Carbon Monoxide Co Click On The Pair Of Electrons That Is Most Consistent With Lone Pair Of Electrons Localized

Draw The Molecular Orbitals For Co In Order Of Energy And Fill Them With The Appropriate Number Of Electrons Label The Orbitals The Best You Can As Sigma Or Pi And As
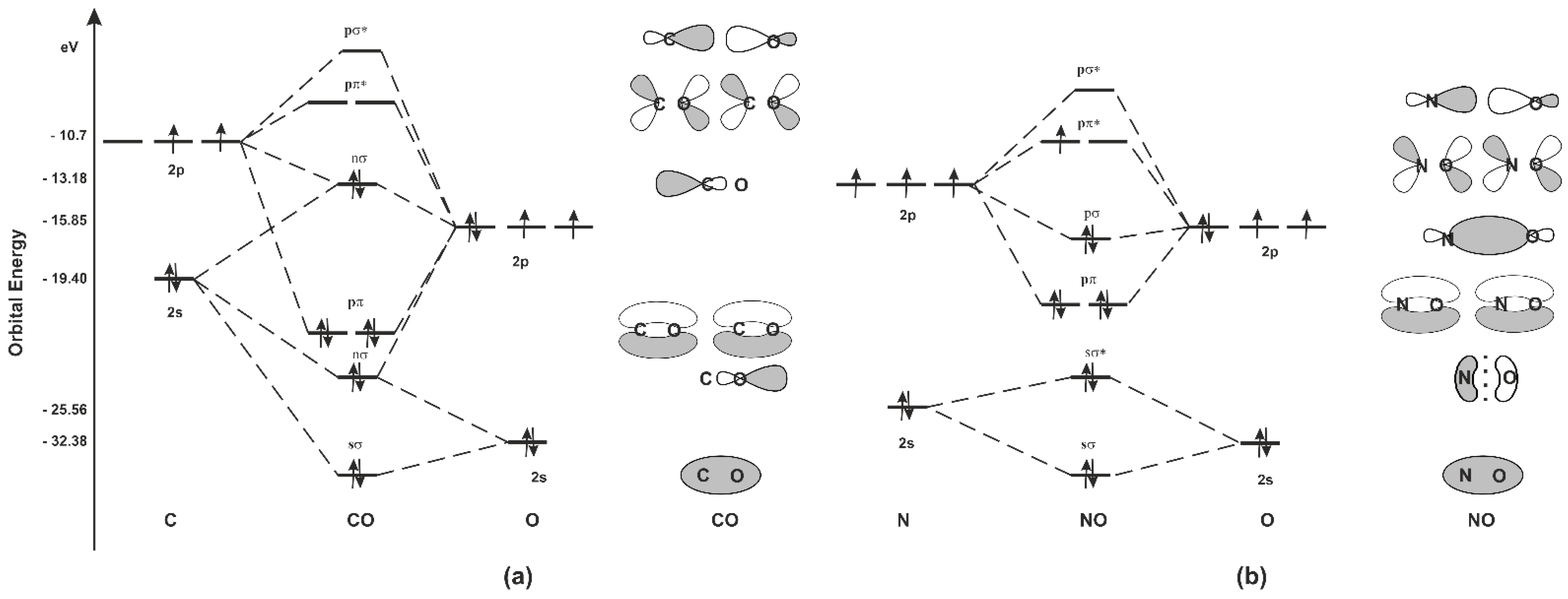
Ijms Free Full Text Carbon Monoxide And Nitric Oxide As Examples Of The Youngest Class Of Transmitters Html


















Comments
Post a Comment