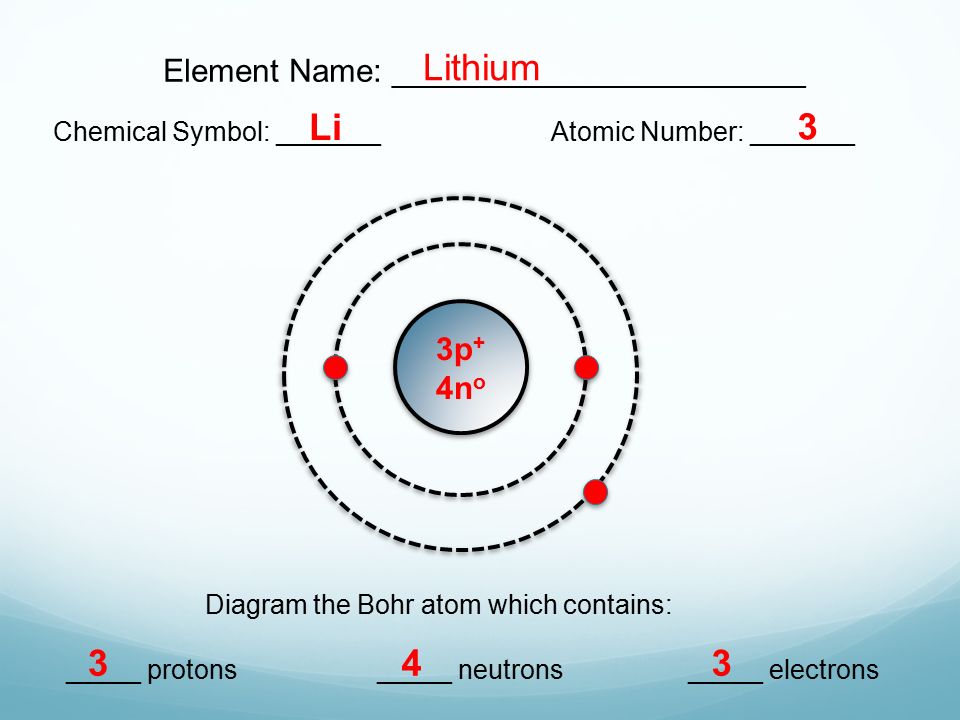
42 li bohr diagram
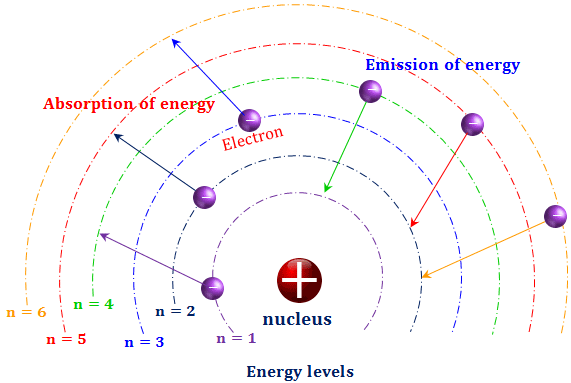
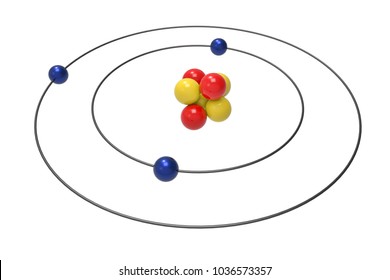
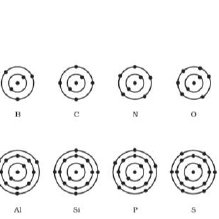
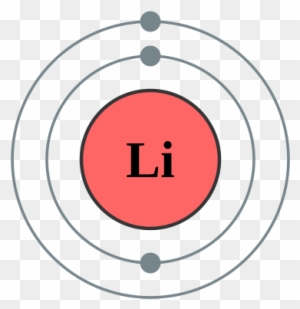
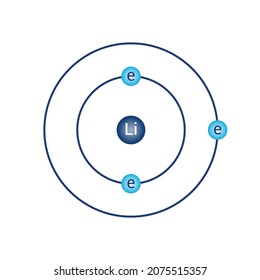
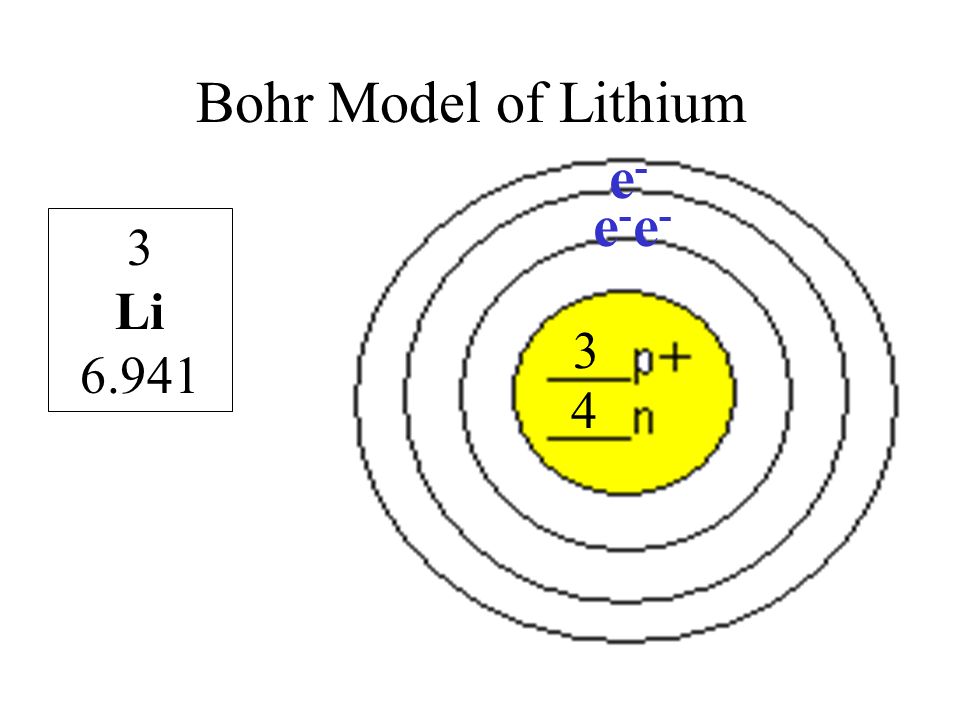
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Lithium has three electrons: two go to K shell and. A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels.
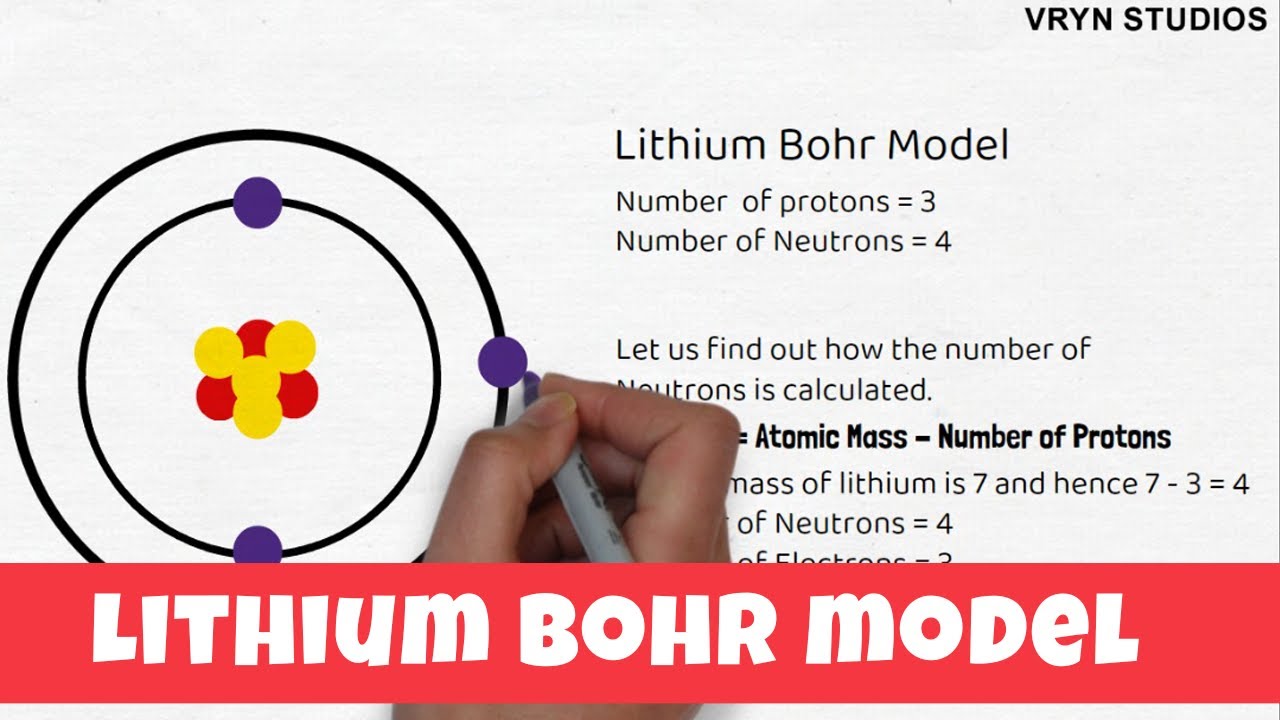
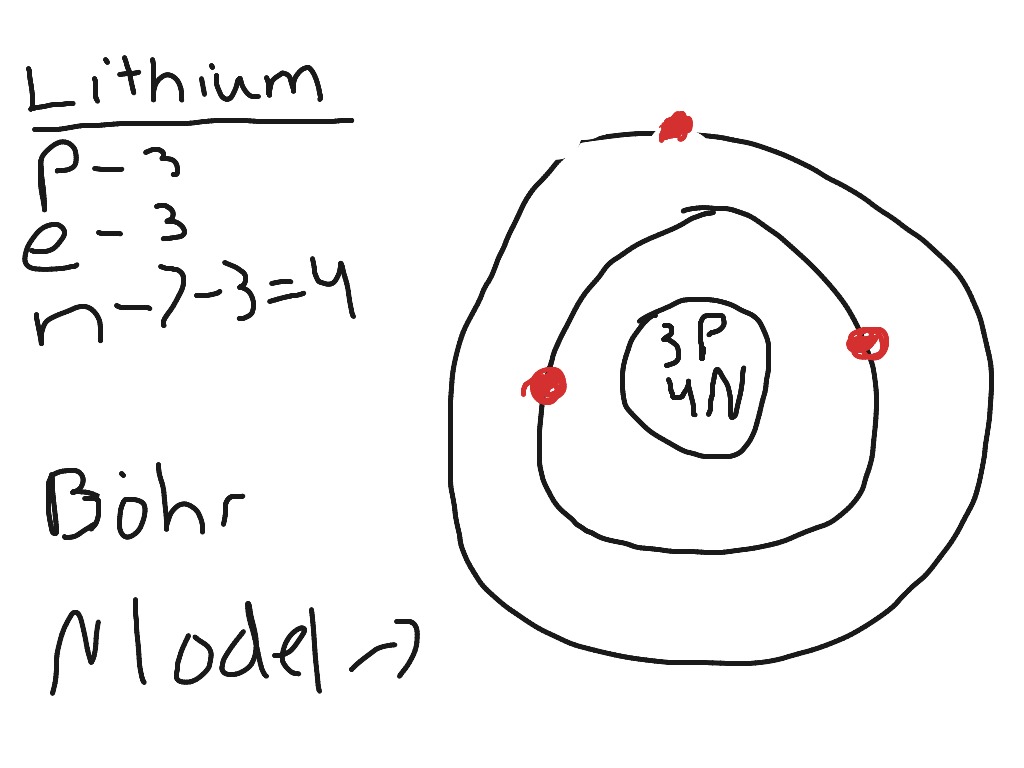
Our new Bohr model has suceeded in calculating the Helium ionization energy more correctly than the quantum mechanical variational methods as shown in the Top page. So next, we try Lithium atom (Li) and Lithium ion (Li+) by Bohr's theory. Lithium belongs to the alkali metal group of chemical elements, and has the atomic number 3.
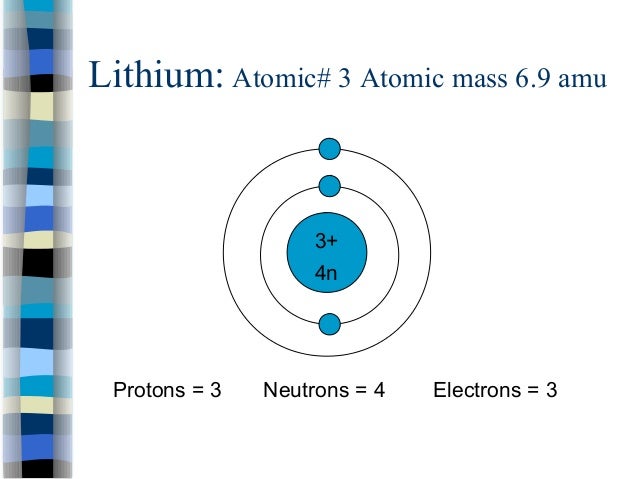
Li bohr diagram
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. How do Bohr models work? Just so, what is the Bohr diagram for lithium? Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Lithium has three electrons: two go to K shell and. A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Jan 18, · Aluminum, Al Bohr Diagram.
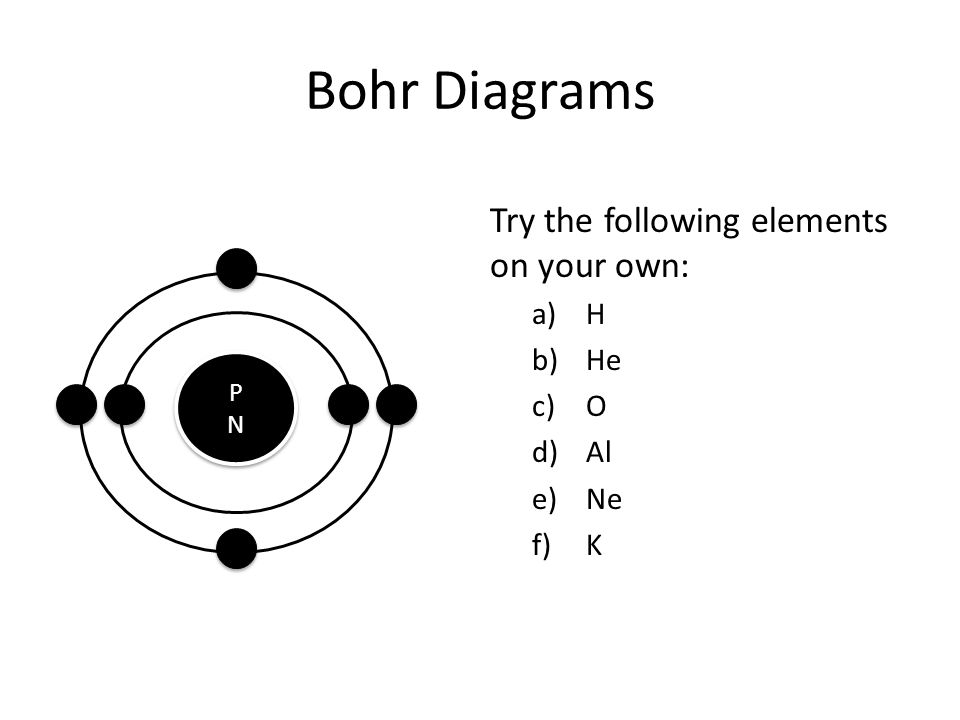
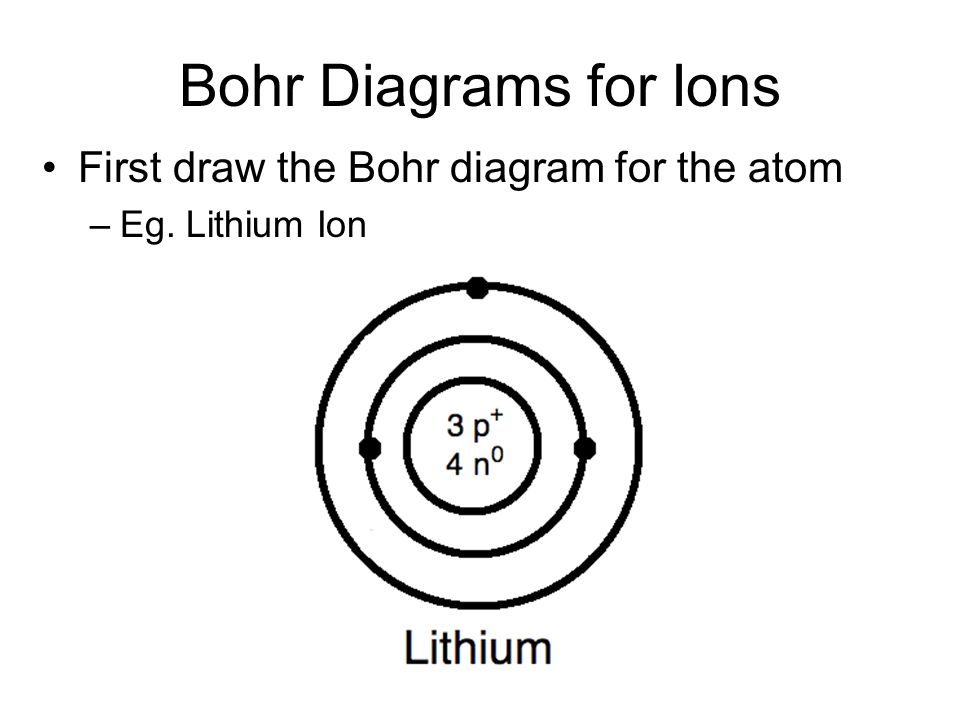
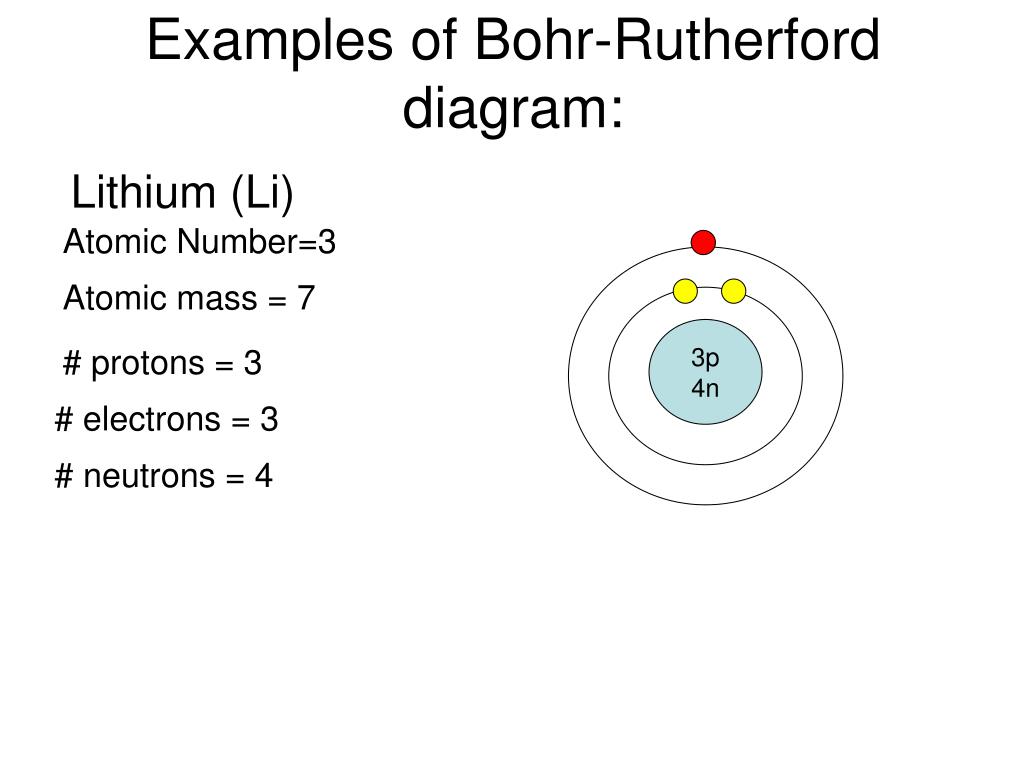
Li bohr diagram. 3.3.1a - Bohr Diagram. Let's take a look at how to draw Bohr diagrams: For a hydrogen atom, H, the one electron goes into the first energy level. Draw a circle and label it with the symbol of the nucleus, H. Write the number of protons for the nucleus, 1p +. Draw an arc to represent the first energy level. Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n Nov 18, 2018 · In atomic physics, the Rutherford–Bohr model or Bohr model or Bohr diagram, presented by Niels Bohr and Ernest Rutherford in , is a system consisting of a small, dense nucleus surrounded by revolving electrons —similar to the structure of.draw a Bohr-Rutherford diagram for lithium. draw a Bohr-Rutherford diagram for beryllium. draw a Bohr ... This video explains on the atomic model structure of Lithium. This is a "Bohr model" on Lithium. #lithium #bohr_model #howtodraw #atomic_model
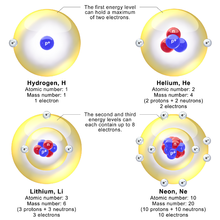
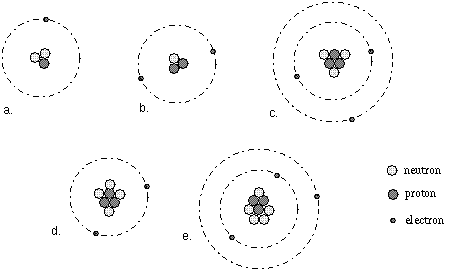
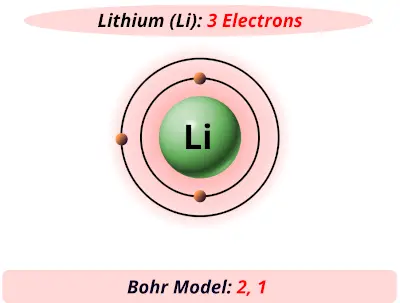
The Bohr Model of Potassium(K) has a nucleus that contains 20 neutrons and 19 protons. This nucleus is surrounded by four-electron shells named K-shell, L-shell, M-shell, and N-shell. The outermost shell in the Bohr diagram of Potassium contains only 1 electron that also called valence electron. Bohr model of Hydrogen (H) 1: 2: Bohr model of Helium (He) 2: 3: Bohr model of Lithium (Li) 2, 1: 4: Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11 ... lithium bohr diagram. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. And in the orbit of Fig. the wavelength of the photon given off is given by. So approximately, we can suppose the 2S electron is moving around the e+ nucleus ( +3e - 2e = +e ) on the circular orbit of the two de Broglie ... P=___ N=___ E=___ O S O L O G Al _____ _____ M.P. = _____ B.P. = _____ Properties Uses Al Bohr Diagram Lewis Structure P=___ N=___ E=___ O S O L O G Li _____ _____ M ...
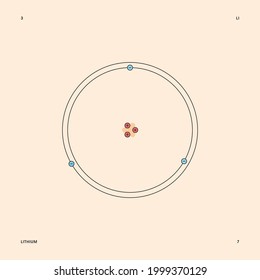
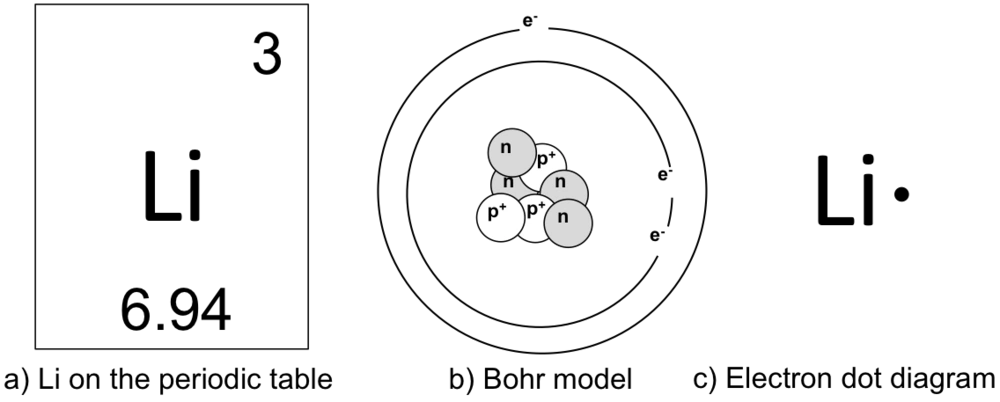
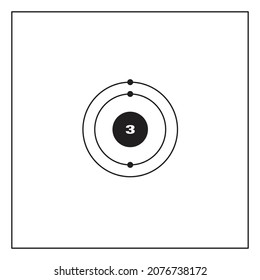
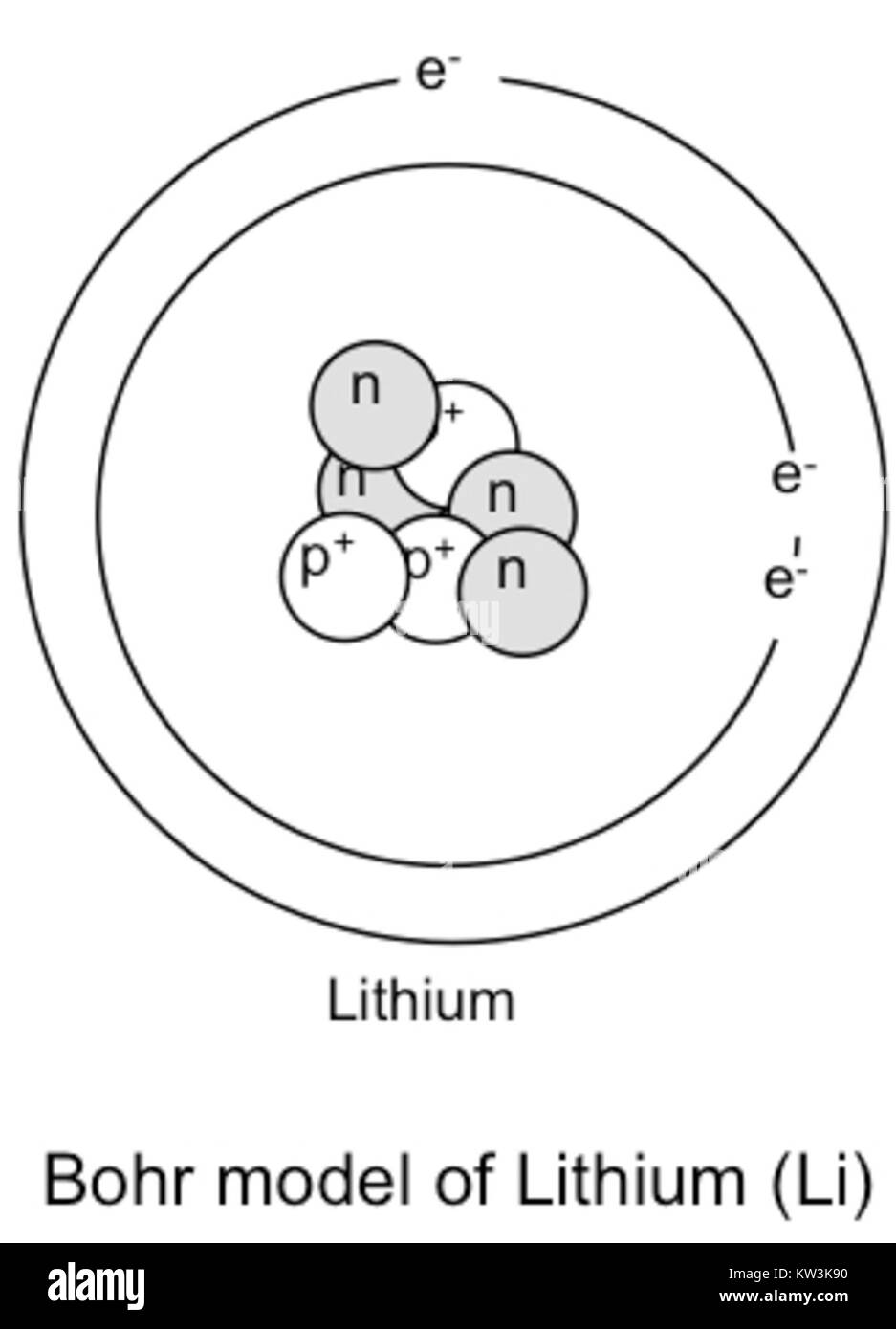
Lithium may also be absorbed via the lungs. A systemic resorption of lithium was shown in a study on 27 intensive care unit patients, who were mechanically ventilated with lithium-chloride-coated heat and moisture exchangers for at least 5 days. Serum lithium was non-detectable at the first measurement, whereas 0.01-0.05 mM appeared in the blood from the 1st to the 4th day. Determining the lonic Charge After Making an Octet Element Property Before Making an Octet electron config " protons Relectrons chargePoSitV Li Bohr Diagram Lewis Dot Structure electron confi # protons #electrons rpや Be Bohr Diagram Lewis Dot Structure electron contig # protons #electrons charge 8 Bohr Diagram Lewis Dot Structure Skip Carborn. What is the pattern for the first 4 she…. Shows how many electrons each shell has. Niels Bohr. The number of protons and neutrons are shown in the centre and…. 2, 8, 8, 18. Bohr Diagram. Shows how many electrons each shell has. Named after. Niels Bohr. Bohr's diagram of Lithium has only two electron shells (K and L), the inner shell is K-shell and the outermost shell is L-shell. Hence, the electrons found in the L-shell of the Lithium atom are its valence electrons because it is the outermost shell that also called the valence shell.
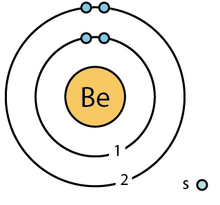
Bohr Diagrams So far we have used Bohr diagrams to show how may electrons an element has and how elements bond in ionic compounds. For example here is CaF 2. ... Lithium Hydride Electron dot diagrams can be used to show the formation of ionic compounds. Li has 1 valence electron. H also has 1 valence electron.
See answer (1) Best Answer. Copy. Li in the centre, then draw two circles around it, then put two electrons on the inner circle and then 1 electron on the outside. Wiki User. ∙ 2009-08-28 19:12 ...
A step by step program for creating Bohr Diagrams and Electron dot structures. Usefule for Index Cards and Flash cards for chemistry and physical science stude…
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Jan 18, · Aluminum, Al Bohr Diagram.
Just so, what is the Bohr diagram for lithium? Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Lithium has three electrons: two go to K shell and.
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. How do Bohr models work?














Comments
Post a Comment