40 lewis electron dot diagram for fluoride ion
What is the lewis dot structure for Fluoride, F1- and Mg2??? What is the lewis dot structure for copper? Chemistry. What is the three-dimensional structure of the nuclei of atoms in a molecule or polyatomic ion called? Lewis Dot structure bond length molecular geometry molecular formula Won't it be Molecular geometry . View more similar questions or ask a new question. What is the Lewis electron-dot diagram for... | Clutch Prep FREE Expert Solution. In order for us to draw the electron dot diagram of fluoride, we must first determine the number of valence electrons of it. Fluorine can be seen in Group 7A elements which have 7 valence electrons on it. 84% (176 ratings) Sign up for free to view this solution. Sign up for free.
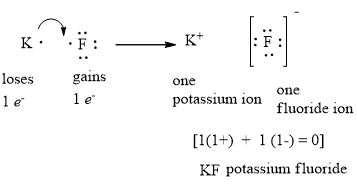
Lewis Electron Dot Diagram For Fluoride Ion Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon.The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Examples. Lithium fluoride, LiF.

Lewis electron dot diagram for fluoride ion
Lewis Electron Dot Diagram For Fluoride Ion - schematron.org Mar 09, 2018 · The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Lithium fluoride, LiF 1. Lithium atom loses one electron to form the cation Li + 2. Fluorine atom gains one electron to form the anion F Lithium fluoride compound can be represented as Li + OR 1. How to Draw the Lewis Dot Structure for NaF: Sodium fluoride A step-by-step explanation of how to draw the NaF Lewis Dot Structure.For NaF we have an ionic compound and we need to take that into account when we draw th... Lewis Electron Dot Structures - Detailed Explanation with ... The resulting Lewis electron dot structure displays a triple bond connecting a carbon and an oxygen atom, each holding a lone pair of electrons. Solved Examples. Problem-1: In terms of electron dot formulas, define the electron structure of the carbonate ion CO 3 2-. Solution: One potential electron dot formula for the carbonate ion is
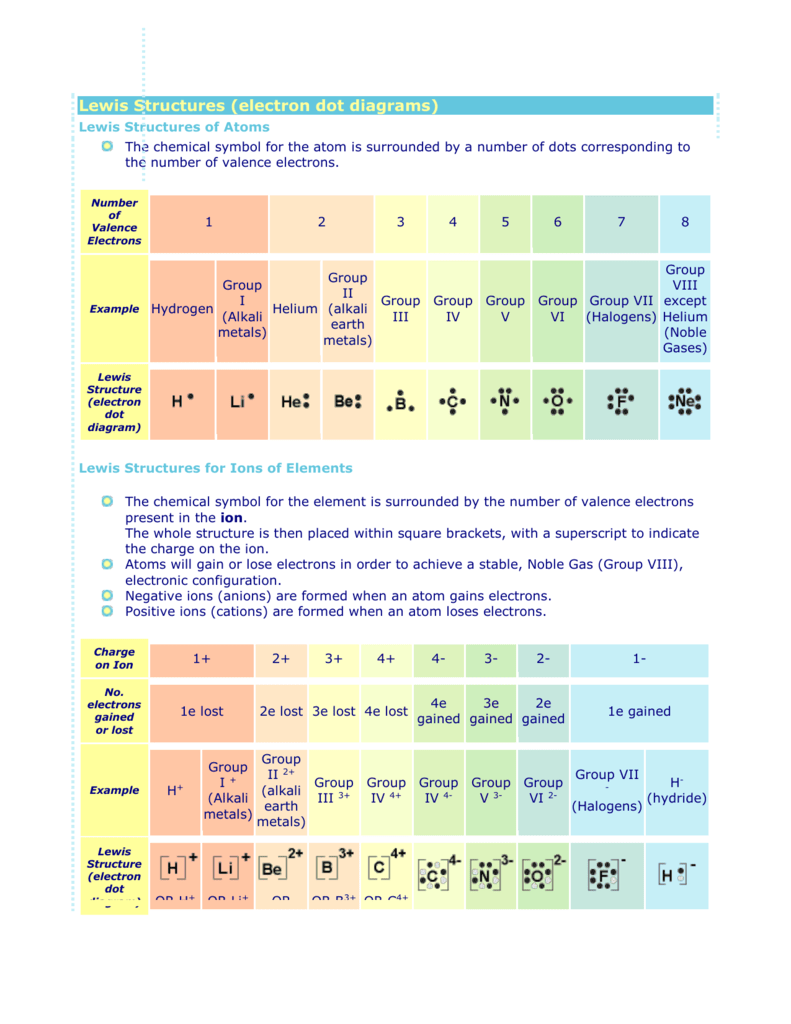
Lewis electron dot diagram for fluoride ion. Which Lewis Dot Diagram Represents A Fluoride Ion A fluoride ion has the same electronic structure as a neon atom (Ne). Answer: Fluorine is in Group 17 of the Periodic Table. Which do you think would be bigger; fluorine atom or fluoride ion?. Draw a Lewis electron dot diagram for an atom or a monatomic ion. a simple way of representing those valence electrons would be useful. Fluoride-Neon. 31 ... potassium fluoride lewis dot structure - ICC A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The bifluoride on heating yields potassium fluoride: Platinum or heat resistant plastic containers are often used for these operations. NO2F Lewis Structure, Molecular Geometry, Hybridization ... NO2F Lewis Structure, Molecular Geometry, Hybridization, and Polarity. NO2F, an inorganic compound, is named Nitryl Fluoride. It is a colorless gas and acts as a strong oxidizing agent. So, it can be used in the replacement of liquid oxygen, an oxidant in propellants of the rocket. NO2F can easily release its fluoride ion to other species in ... Which of the following is the Lewis dot structure for the ... 2. Draw the Lewis structure for each of the following ions: a. N3-b. Ga3+ c. S2-d. O2-3. Classify the following compounds as ionic (I) or covalent (C): a. FeCl2 b. NO2 c. CO d. AlP. 4. Give the Lewis structure for each of the following: a. NF5 b. ClO2-c. SO3 d. MgCl2. 5. Give the most probable ion formed from each of the following elements: a ...
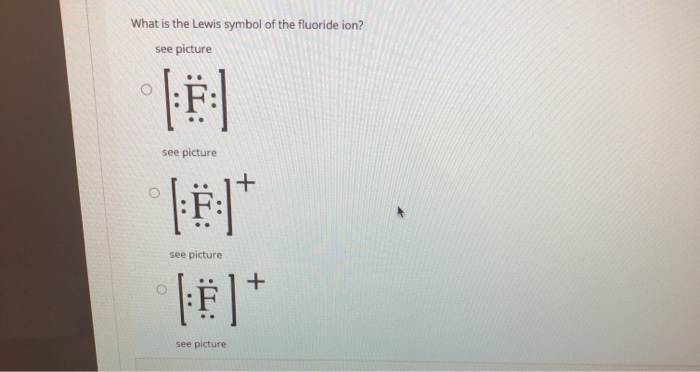
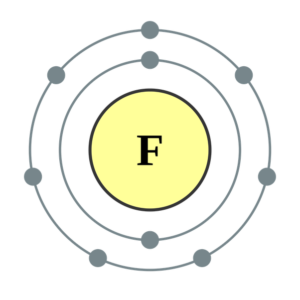
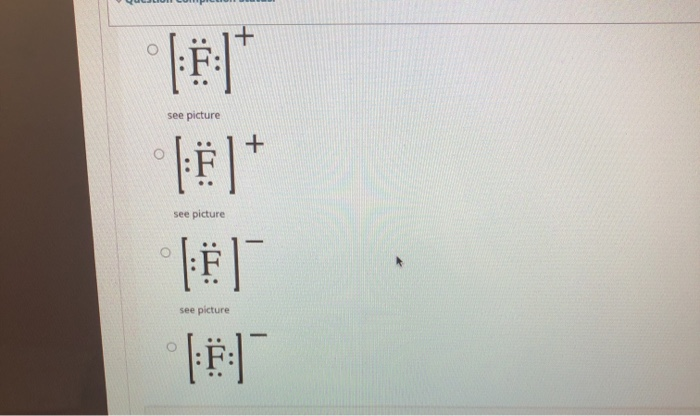
Regents Chemistry Exam Explanations August 2016 OR. A lithium ion has one fewer electron. 62 In the space in your answer booklet, draw a Lewis electron-dot diagram for a fluoride ion. [1] Link to answer. 63 Describe the general trend in atomic radius as each element in Period 2 is considered in order from left to right. [1] Answer--> The atomic radius gets smaller. on to Questions 64-65. allwebitalia.it 21.02.2022 · Worksheets Lewis Dot Structures For each of the following, draw the Lewis Dot Structure, give the electron arrangement (E. Recognizing the showing off ways to acquire this book student exploration ionic bonds answer key is additionally useful. Compare and contrast ionic bonds and cova-lent bonds. When drawing Lewis Dot Structures for ionic compounds … PDF Lewis structure electron-dot formula share A Lewis structure (or electron-dot formula) ... are held together because they share one or more pairs of electrons. For example, the Lewis structure for the hydrogen fluoride (HF) molecule is shown below: H F ... Case 2: Draw the Lewis electron-dot structure for nitrite ion, NO2 ... Solved Writing Lewis Structures for Elements 1) Which of ... 1) Lewis dot structure of Fluoride ion #Valence electrons in F = 7 #Valence electrons in F- = 7+1 = 8 thus the lewis dot structure of fluoride ion (F-) will have 8 electrons around F and a negative charge option e is correct 2) Lewis dot structure of…
How to Draw the Lewis Dot Structure for F- (Fluoride ion ... A step-by-step explanation of how to draw the F- Lewis Dot Structure.For the F- Lewis structure use the periodic table to find the total number of valence el... Lewis Dot Diagram For Fluorine A Lewis structure or Lewis dot diagram, represents the bonds formed between two . Lewis Dot notation is a way of describing the outer shell (also called the valence shell) of an atom's electrons. Dots are Examples are Fluorine and Sulfur. Lewis The ions are arranged in a crystalline structure with each Na+ ion attracted to. 9.2: Lewis Electron Dot Diagrams - Chemistry LibreTexts A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. hemadri.pl 24.02.2022 · Fluoride acid is widely used as Every electron-pair donors that are able to form compounds with the binding of transitional elements can be termed as Lewis bases. Thus, the H-BR bond is weaker than the H-F bond and Dr- is more stable than F-. 38 (Mean or Weighted MP) VP(mm Hg,25 deg C): 9E-009 (Modified Grain method What is the name of the compound H3P …
What is the Lewis electron-dot diagram for a fluoride ion ... What is the Lewis electron-dot diagram for a fluoride ion? Chemistry Covalent Bonds Drawing Lewis Structures. 1 Answer anor277 Jan 7, 2017 Fluorine is in Group 17 of the Periodic Table..... Explanation: And thus the neutral atom has 7 valence electrons. Of course the elemental form is bimolecular.
What is the correct lewis electron-dot structure for the ... As we know that phosphorous has '5' valence electrons and hydrogen has '1' valence electron. Therefore, the total number of valence electrons in phosphorus trihydride, = 5 + 3(1) = 8. According to Lewis-dot structure, there are 6 number of bonding electrons and 2 number of non-bonding electrons.
What Is The Correct Lewis Electron-Dot Structure For The ... Lewis symbol for magnesium ion has no dots and a +2 charge. ; this means one additional electron compared to a fluorine atom. Lewis symbol for fluoride ion has 8 dots and a -1 charge. What is the Lewis structure for magnesium and sulfur? The Lewis dot structure for Magnesium is an Mg with 2 dots which stand for its two valence electrons.
What is the Lewis dot structure of bicarbonate ion? - Answers The Lewis Dot structure of Cyanide ion starts with a C atom connected to a N atom with three dashes. On the opposite sides on each atom are two dots for the unshared valence electrons.
potassium fluoride lewis dot structure - ofcs.org The Lewis Structure (electron dot diagram) of each ion is used to construct the Examples Lithium fluoride, LiF Lithium atom loses one electron to form the. Like other sources of the fluoride ion, F−, KF is poisonous, although lethal doses approach gram levels for humans.
Lewis Dot Diagram For Fluorine - schematron.org A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. atoms. We do this by forming what are called Lewis diagrams.
PDF Scanned with CamScanner Lewis Dot ms, Atoms and Ions 9. Which electron-dot symbol represents an atom of in the ground state? Ar: B) 'AF: C) A) 10. Which Lewis diagram represents ax fluoride ion? I. Which Lewis lectron-dot diagram represe nifrogen atom in the ground state? 5 2. Which Lewis electron-dot diagram represents an atom in the ground state for a Group 13 ...
Electron Dot Diagram For Fluorine - Wiring Diagrams Lewis Structure (electron dot diagram) for hydrogen fluoride OR The 2 electrons making up the bonding pair of electrons between the hydrogen atom and the fluorine atom, which may or may not be circled, are referred to as a covalent bond (or a single covalent bond).Electron Dot StructuresWhat is the Lewis dot structure for fluorine.
Lewis Structures or Electron Dot Structures Lewis structures, also known as electron dot structures, are named after Gilbert N. Lewis, who described them in a 1916 article titled, "The Atom and the Molecule." Lewis structures depict the bonds between atoms of a molecule, as well as any unbonded electron pairs. You can draw a Lewis dot structure for any covalent molecule or coordination ...
PDF Lewis Dot Structures of Atoms and Ions B. Lewis dot structure for a chloride ion is Chlorine needs an additional electron to attain the stable noble gas configuration of 8 valence electrons. Since chlorine is a nonmetal, it has relatively high values for electronegativity and ionization energy. This means that it will gain electrons until it achieves a stable octet.
How to Draw the Lewis Dot Structure for LiF: Lithium fluoride step-by-step explanation of how to draw the LiF Lewis Dot Structure.For LiF we have an ionic compound and we need to take that into account when we draw the ...
Lewis Structures (electron dot diagrams) Chemistry Tutorial Lewis Structure (electron dot diagram) for hydrogen fluoride OR The 2 electrons making up the bonding pair of electrons between the hydrogen atom and the fluorine atom, which may or may not be circled, are referred to as a covalent bond (or a single covalent bond).
Lewis Electron Dot Structures - Detailed Explanation with ... The resulting Lewis electron dot structure displays a triple bond connecting a carbon and an oxygen atom, each holding a lone pair of electrons. Solved Examples. Problem-1: In terms of electron dot formulas, define the electron structure of the carbonate ion CO 3 2-. Solution: One potential electron dot formula for the carbonate ion is
How to Draw the Lewis Dot Structure for NaF: Sodium fluoride A step-by-step explanation of how to draw the NaF Lewis Dot Structure.For NaF we have an ionic compound and we need to take that into account when we draw th...
Lewis Electron Dot Diagram For Fluoride Ion - schematron.org Mar 09, 2018 · The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Lithium fluoride, LiF 1. Lithium atom loses one electron to form the cation Li + 2. Fluorine atom gains one electron to form the anion F Lithium fluoride compound can be represented as Li + OR 1.


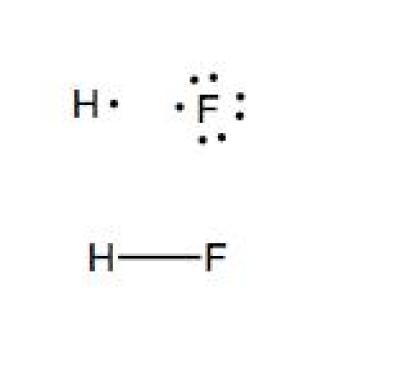












/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)



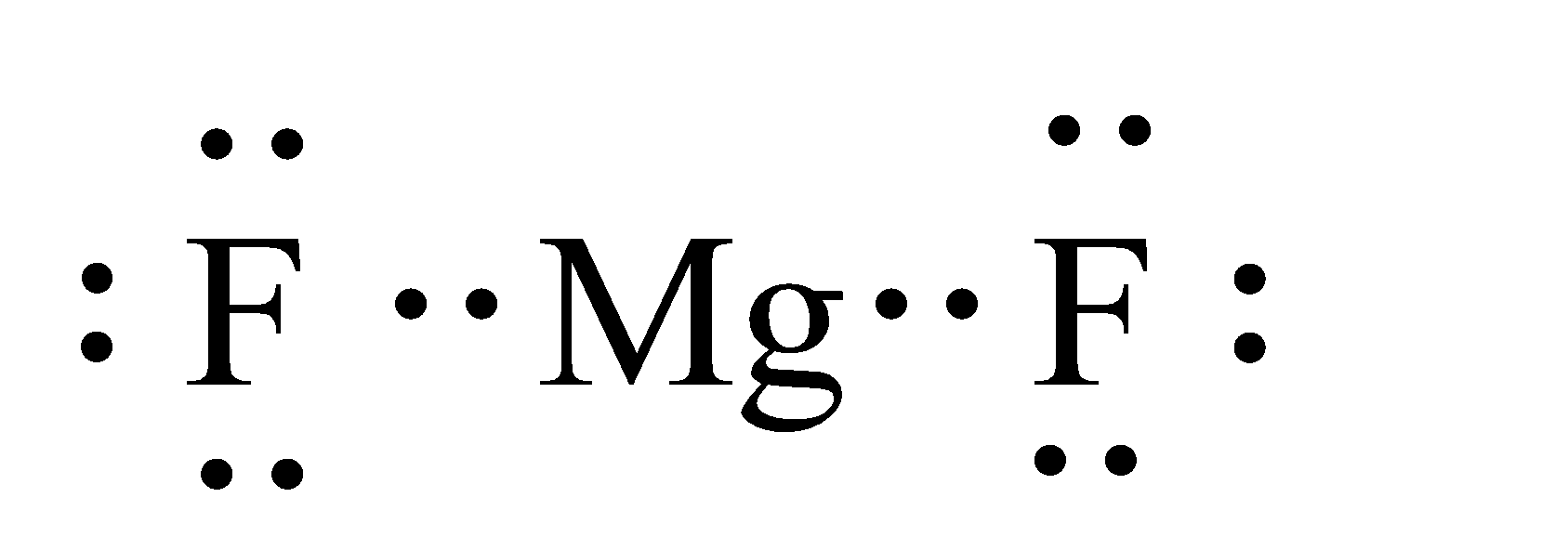





Comments
Post a Comment