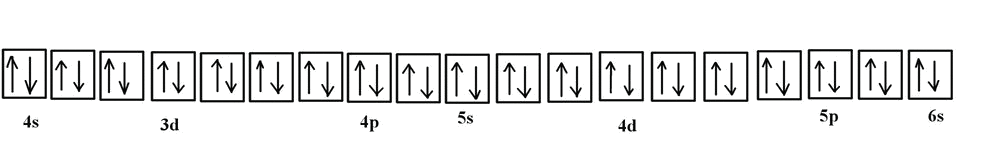
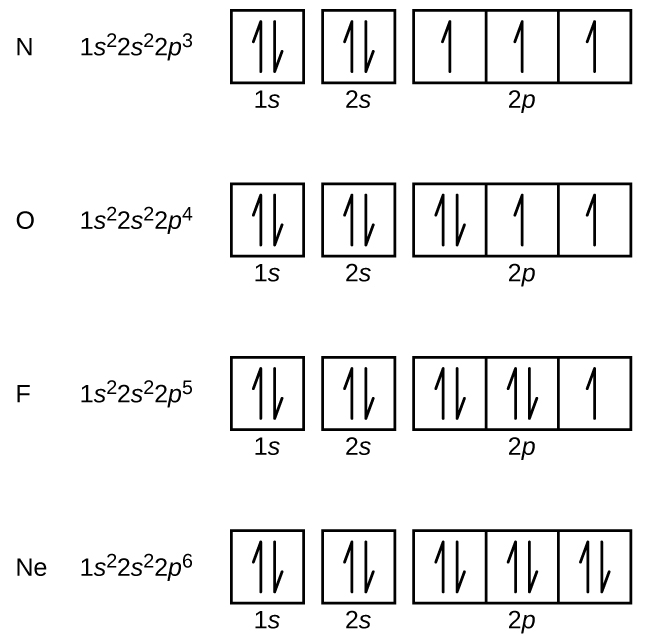
39 orbital diagram for silver
Silver, electron configuration - prvky Silver. Full electron configuration of silver: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1. palladium ← silver → cadmium. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
WebElements Periodic Table » Silver » crystal structures β: 90.000°. γ: 90.000°. You may view the structure of silver: interactively (best, but the page will take longer to load) or. non-interactively. Silver crystal structure image (ball and stick style). Silver crystal structure image (space filling style).

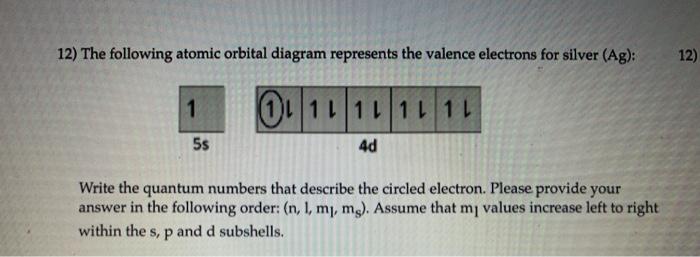
Orbital diagram for silver
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram of lithium The electron configuration of lithium is 1s²2s¹. This means that there are two electrons in the 1s orbital, and one electron in the higher energy 2s orbital. If you want to make a cool picture, you can do it like this: Solved 6. Draw the orbital energy (i.e., box) diagram for ... Draw the orbital energy (i.e., box) diagram for the electrons beyond the [Kr) core of the element silver (Ag). If all the electrons are paired in the orbital energy diagram, the element is said to be diamagnetic, meaning that atoms of the element will interact weakly with an external magnetic field. The Electron Configurations of Atoms - Chemistry at Illinois A. Box Diagrams of Electron Configuration If an atom has a partially filled sublevel, it may be important to know how the electrons of that sublevel are distributed among the orbitals. Research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an ...
Orbital diagram for silver. Silver(Ag) electron configuration and orbital diagram This is clearly shown in the figure of the orbital diagram of silver. Silver ion (Ag +) electron configuration Ground state electron configuration of silver is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 1. This electron configuration shows that the last shell of silver has an electron and the d-orbital has a total of ten electrons. 3.4 Electronic Structure of Atoms (Electron ... - OpenStax The 15 electrons of the phosphorus atom will fill up to the 3 p orbital, which will contain three electrons: The last electron added is a 3 p electron. Therefore, n = 3 and, for a p -type orbital, l = 1. The ml value could be -1, 0, or +1. The three p orbitals are degenerate, so any of these ml values is correct. Orbitals Periodic Table - breakingatom.com Orbitals and Electron Configuration. The Periodic Table with Oxidation Numbers and Electron Configurations. S Block. P Block. D Block. F Block. 1. Hydrogen. 1. Arrangements of electrons in the orbitals of an atom is ... The orbital diagram for hydrogen can be represented in the following way. This notation uses a box to represent the orbital, the label for the orbital and an arrow to represent the electron. The electronic configuration for hydrogen can be written as 1s 1. This is a short-hand notation which identifies the level, the sublevel and the number of ...
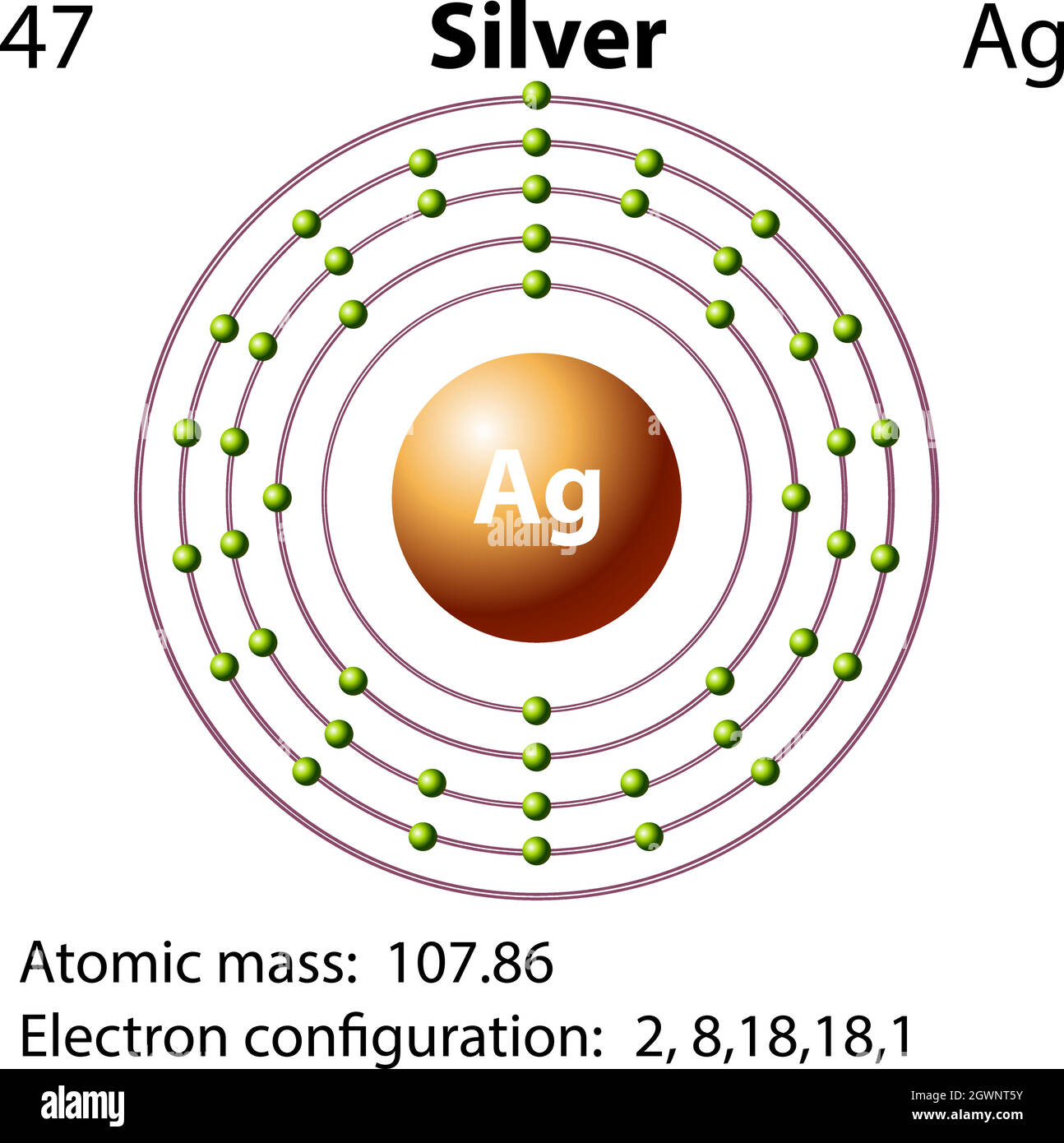
Structure of the Silver Atom | Purest Colloids The silver atom has 5 electron orbits (energy levels) with a total of 47 electrons. Beginning with the orbit closest to the nucleus and working outward, the number of electrons per orbit should be: 2, 8, 18, 18, 1. Of course, the nucleus contains 47 protons and 61 neutrons. How do yo write the orbital diagram for silver? | Socratic Explanation: A quick glance at the Periodic Table tells us that ZAg = 47 ..and so for the NEUTRAL atom.... We use the Aufbau process to write the electronic distribution of the SILVER atom... 1s22s22p63s23p63d104s24p6 electronic configuration of krypton 5s14d10 How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. Yttrium(Y) electron configuration and orbital diagram The Aufbau principle is that the electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital. These orbitals are named s, p, d, f. The electron holding capacity of these orbitals is s = 2, p = 6, d = 10 and f = 14.
Electron configuration for Silver (element 47). Orbital ... Ag (Silver) is an element with position number 47 in the periodic table. Located in the V period. Melting point: 961.9 ℃. Density: 10.49 g/cm 3 . The order of filling the orbitals with electrons in the Ag atom is an exception to the rule. Expected electronic configuration. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d9. Solved 9. Write out the full electron configuration for ... Write the atomic orbital diagram for the valence electrons of silver and identify if it is paramagnetic or diamagnetic. Write out a possible set of quantum numbers for a valence d-orbital electron of silver Determine the radius of a silver atom (in picometers) given that it crystallizes as a body Question: 9. Silver Valence Electron - rhodium periodic table and ... Silver Valence Electron. Here are a number of highest rated Silver Valence Electron pictures upon internet. We identified it from honorable source. Its submitted by handing out in the best field. We take on this nice of Silver Valence Electron graphic could possibly be the most trending subject following we part it in google benefit or facebook. What is the orbital notation of silver? - Answers What is the electron orbital notation of Ag? Ag, or silver, has the atomic number 47. Its orbital notation is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10.
How Many Valence Electrons Does Silver Have? Facebook. Twitter. Silver has one valence electron. Silver is a brilliant white precious metal with atomic number 47 and 47 electrons arranged in orbits around the nucleus of the atom. The electron configuration of silver is [Kr] 4d10 5s1 or 2, 8, 18, 18 and 1. Silver is a solid at room temperature. It melts at 1,763.2 degrees Fahrenheit.
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Silver - Wikipedia Silver is a chemical element with the symbol Ag (from the Latin argentum, derived from the Proto-Indo-European h₂erǵ: "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. The metal is found in the Earth's crust in the pure, free elemental form ("native silver ...
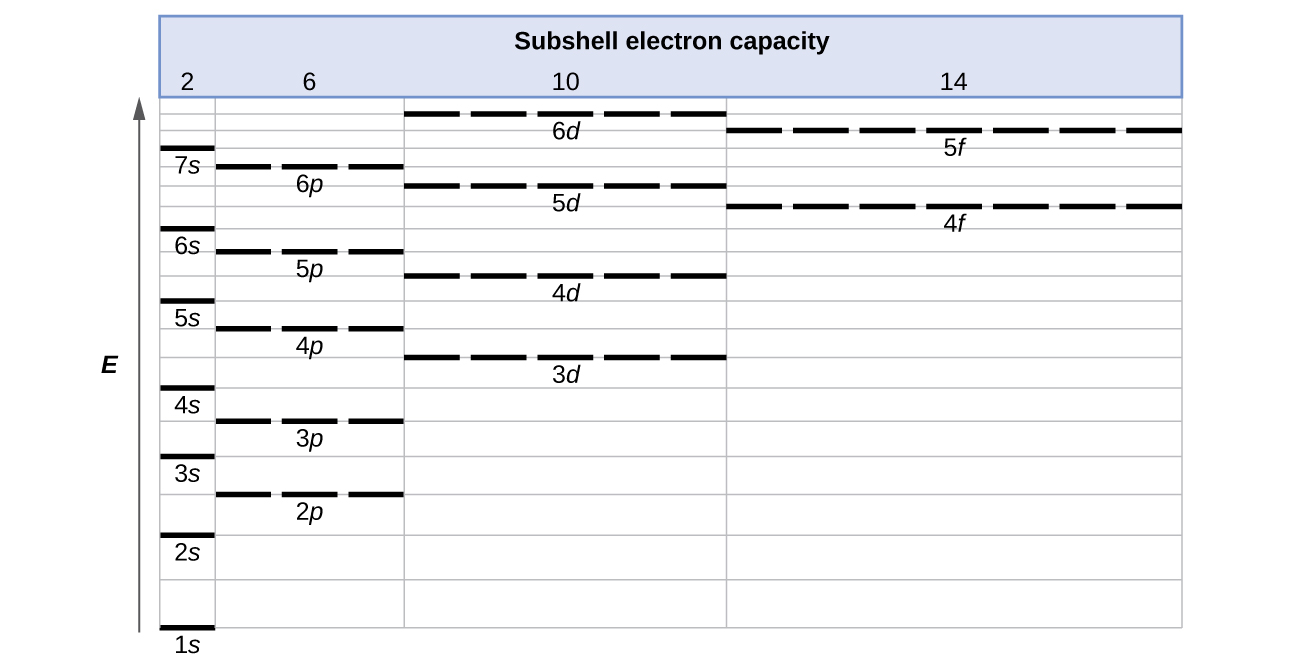
PDF Shells, Subshells, and Orbitals - The SHAPE of an orbital is defined by the SUBSHELL it is in - The ENERGY of an orbital is defined by both the SHELL the orbital is in AND the kind of SUBSHELL it is in ARRANGEMENT OF SHELLS, SUBSHELLS, AND ORBITALS - Shells are numbered. Each shell can contain the same number of SUBSHELLS as its number: 1st shell: ONE possible subshell (s)
Silver (Ag) - ChemicalAid Silver (Ag) has an atomic mass of 47. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
Write the orbital configuration for silver. | Study.com Answer and Explanation: 1 Become a Study.com member to unlock this answer! Create your account View this answer To create the orbital configuration, we can refer to the orbital diagram below. As...
Silver - Element information, properties and uses ... Silver bromide and iodide were important in the history of photography, because of their sensitivity to light. Even with the rise of digital photography, silver salts are still important in producing high-quality images and protecting against illegal copying. Light-sensitive glass (such as photochromic lenses) works on similar principles.
What is the orbital diagram of Silver? - Answers An orbital diagram is used to show how the orbitals of a subshell areoccupied by electrons. The two spin projections are given by arrowspointing up (ms =+1/2) and down (ms = -1/2).
What are the possible magnetic quantum numbers of silver? Silver has electronic configuration as [Kr] 4d10 5s1 . The s orbital ( 5s ) has only one electron. And the s orbital here has n = 5 (as it is in the fifth period) and l = 0 . The s orbital is symmetric in space (sphere) which has only one orientation and hence has no "different" orientations each of which is represented by a particular value of ml.
The Electron Configurations of Atoms - Chemistry at Illinois A. Box Diagrams of Electron Configuration If an atom has a partially filled sublevel, it may be important to know how the electrons of that sublevel are distributed among the orbitals. Research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an ...
Solved 6. Draw the orbital energy (i.e., box) diagram for ... Draw the orbital energy (i.e., box) diagram for the electrons beyond the [Kr) core of the element silver (Ag). If all the electrons are paired in the orbital energy diagram, the element is said to be diamagnetic, meaning that atoms of the element will interact weakly with an external magnetic field.
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram of lithium The electron configuration of lithium is 1s²2s¹. This means that there are two electrons in the 1s orbital, and one electron in the higher energy 2s orbital. If you want to make a cool picture, you can do it like this:



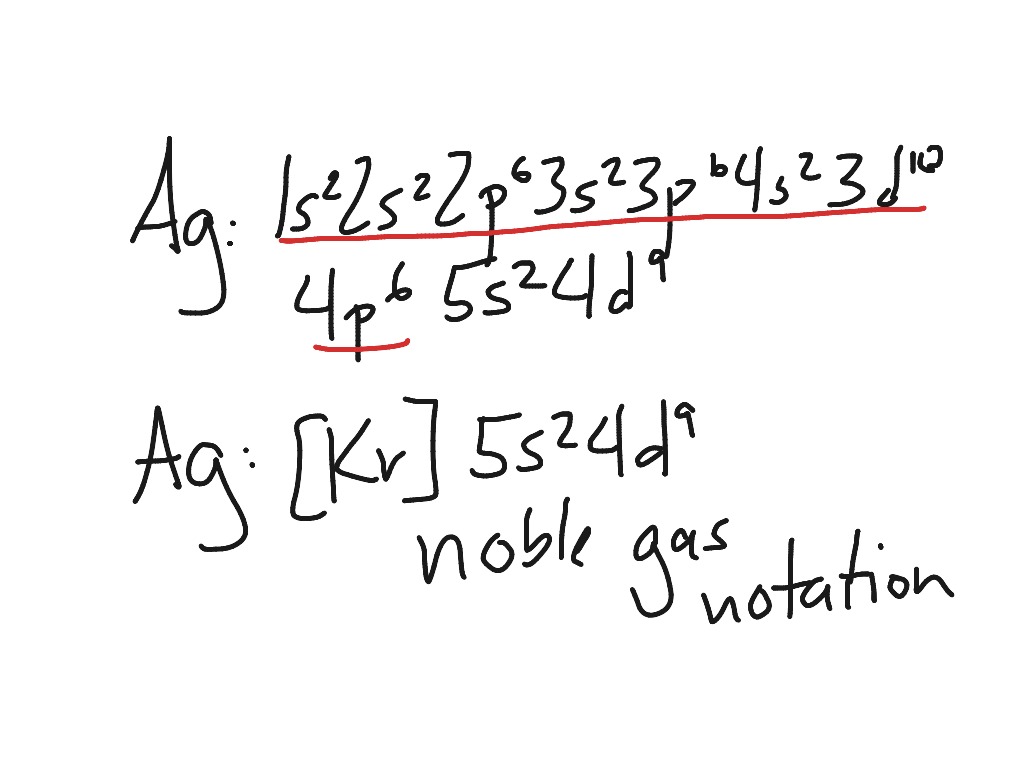



















Comments
Post a Comment