40 enthalpy level diagram
Enthalpy Diagrams - QS Study Enthalpy diagrams are a representation of the relative internal energy of a system before and after a reaction. Horizontal lines are drawn to show the energy of the system at a particular time. The nature of the system which has that energy is written on the line. The formulae of all the elements or compounds present in the system including ... 16 Which statement is correct given the enthalpy level ... 16. Which statement is correct given the enthalpy level diagram below? A. The reaction is endothermic and the products are more thermodynamically stable than the reactants. B. The reaction is exothermic and the products are more thermodynamically stable than the reactants. C.
chemrevise.files.wordpress.com › 2020/04/11.4 Energetics - Resources for A-level and GCSE Chemistry Apr 01, 2020 · Hess’s law states that total enthalpy change for a reaction is independent of the route by which the chemical change takes place Hess’s law is a version of the first law of thermodynamics, which states that energy is always conserved. 2H (g) + 2Cl(g) H2 + Cl2 2HCl (g) a b ΔH On an energy level diagram the directions of the arrows can

Enthalpy level diagram
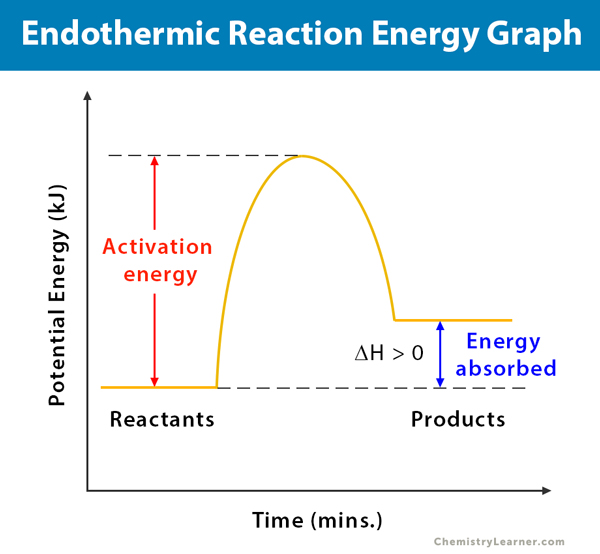
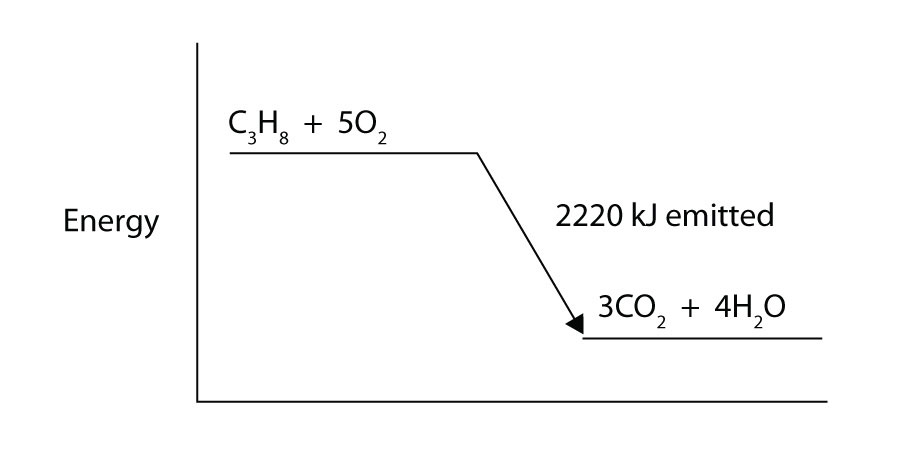
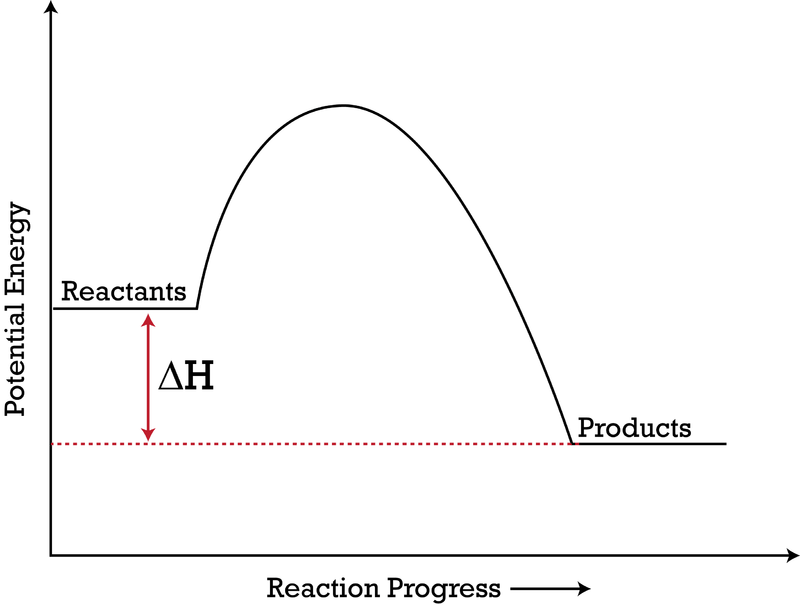
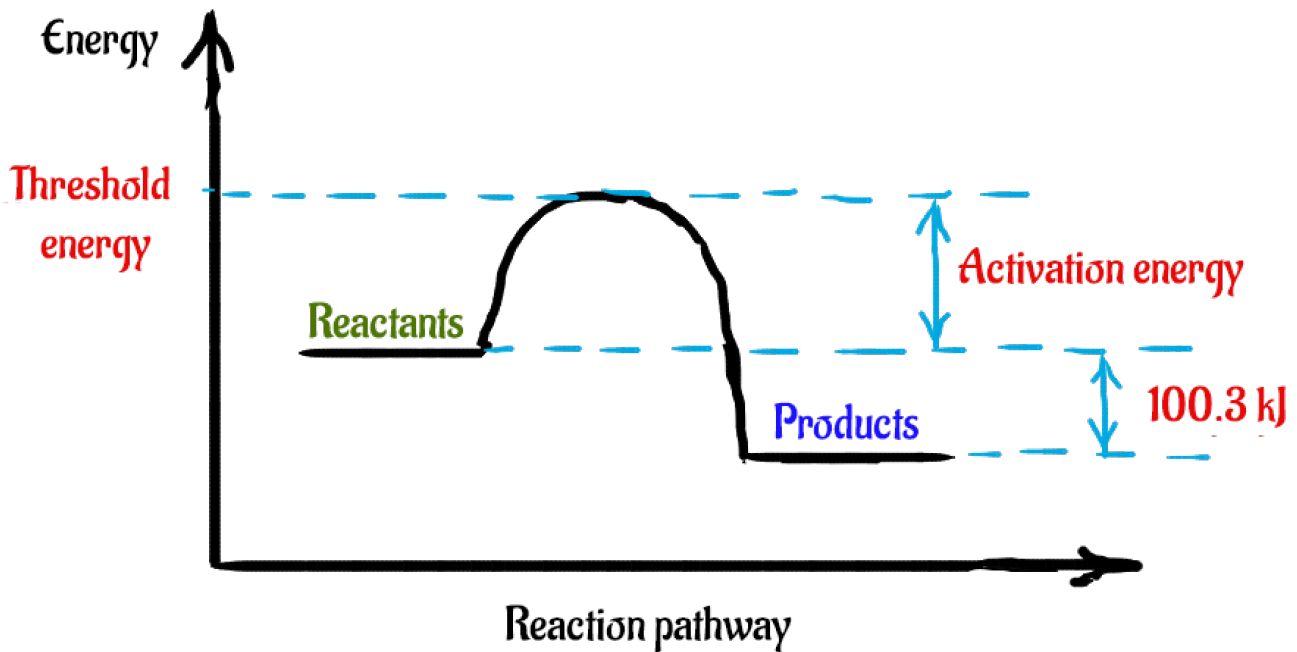
PDF Exothermic enthalpy diagram - Tangente Mag Gives or takes in heat energy, enthalpy change occurs.ΔH = Hproducts - HreactantsWhen petrol is burned enthalpy change is what really matters.ENTHALPY LEVEL DIAGRAMS:For Exothermic reaction ΔH is negative, it means the system loses energy to the surroundings. The products formed will have less energy than the reactants. Enthalpy Level Diagrams (1.8.2) | Edexcel A Level ... Enthalpy Level Diagrams The total chemical energy inside a substance is called the enthalpy (or heat content) When chemical reactions take place, changes in chemical energy take place and therefore the enthalpy changes An enthalpy change is represented by the symbol Δ H (Δ= change; H = enthalpy) An enthalpy change can be positive or negative What is an enthalpy diagram? | Socratic An enthalpy diagram shows the change in enthalpy of the reaction as the chemicals move from reactants to products. The change in enthalpy is a fancy term for the change in thermal energy of the system at constant pressure. Typically we classify reactions as either endothermic or endothermic.
Enthalpy level diagram. (PDF) Enthalpy-concentration Diagram | muhammadichya ... Enthalpy-concentration Diagram • McCabe Thiele method assumes constant molar flow rate because it considers equal latent heat of vaporization. • Here we consider varying molar flow rate by solving simultaneous material and energy balances. • In this case, the operating lines for the enriching and stripping section will be determined from ... Enthalpy - Wikipedia The enthalpy H of a thermodynamic system is defined as the sum of its internal energy and the product of its pressure and volume: H = U + pV, where U is the internal energy, p is pressure, and V is the volume of the system. Enthalpy is an extensive property; it is proportional to the size of the system (for homogeneous systems). 3.2.1 Energetics - Enthalpy change (ΔH) - A level chem On an enthalpy level diagram, higher positions will be less stable (with more internal energy) therefore, if the product is lower, heat is released (more stable, ΔH is -ve) but if it is higher, heat is gained (less stable, ΔH is +ve). Enthalpy level diagrams It is important to get the following facts clear in your mind: Enthalpy-Concentration Diagram - How To Generate ... dH = change in enthalpy = 17449.15 - (-13370.85) = 30820 kJ/kmol** <<-- hooray it agrees. But not quite... Assuming one of the intermediate points; say xA = 0.78, with the reference at 450K. ------------------------- hL = 0.78*138.2* (358.15-450) + (1-0.78)*167.5* (358.15-450) = -13285.735 kJ/kmol
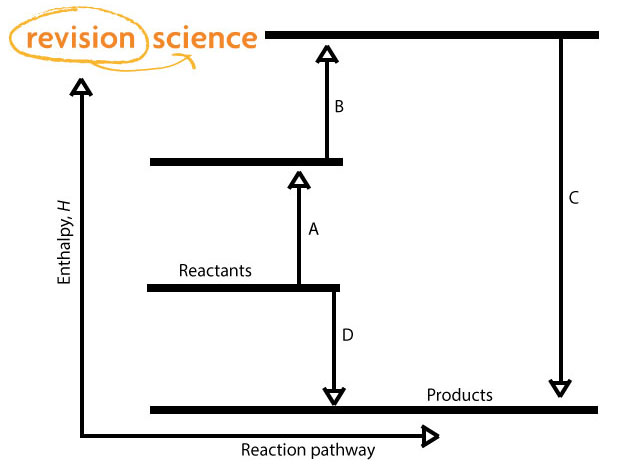
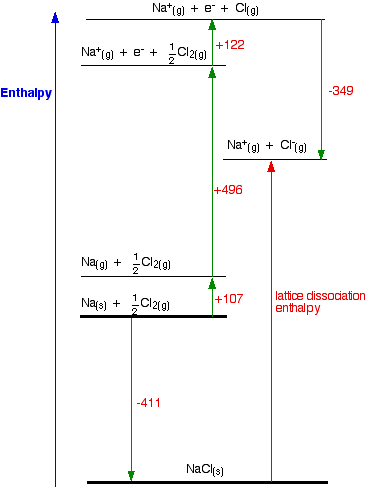
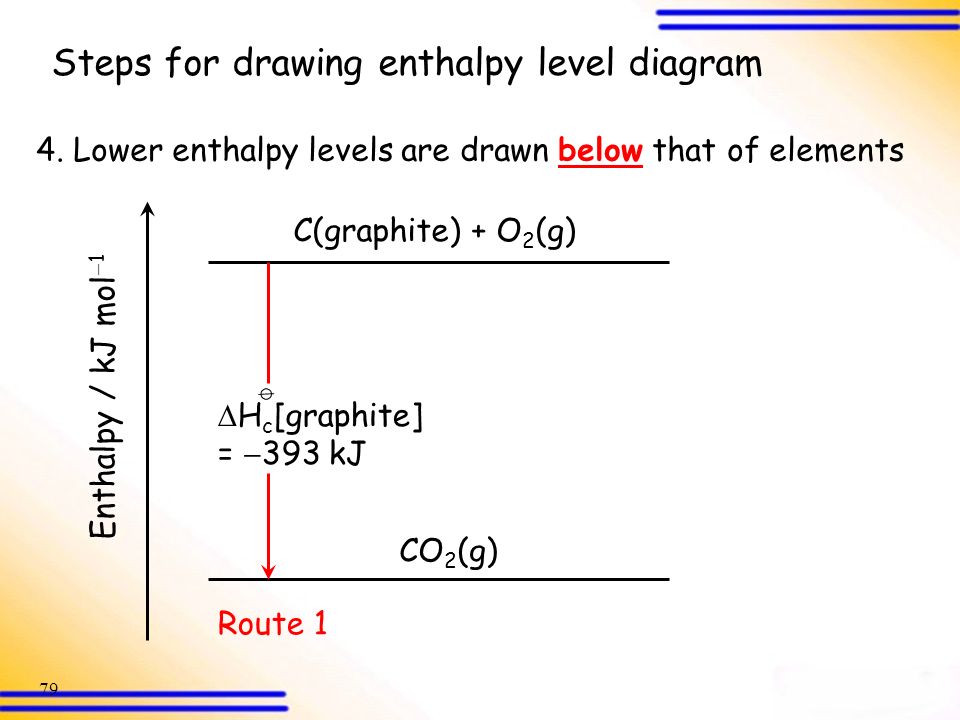
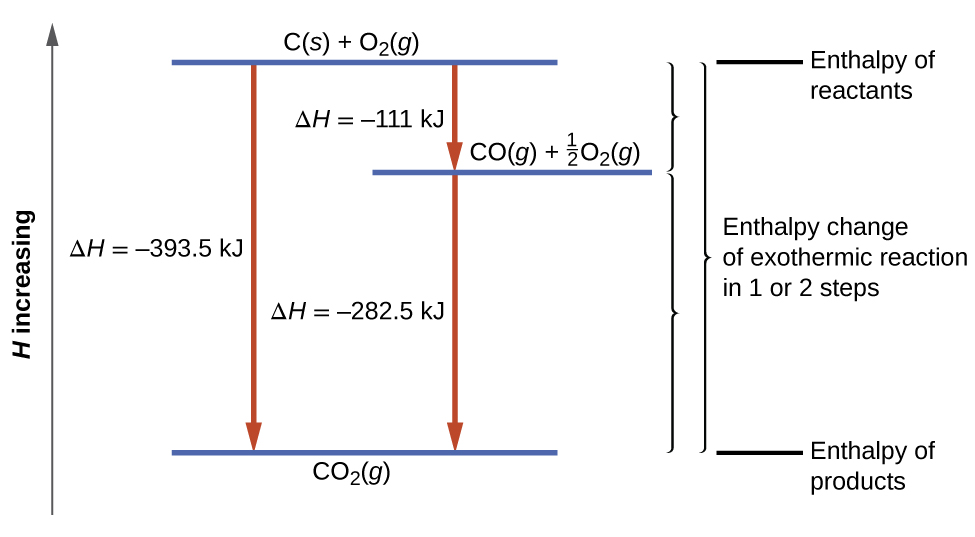
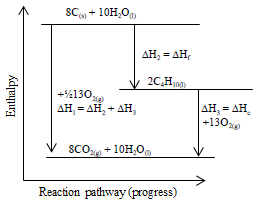
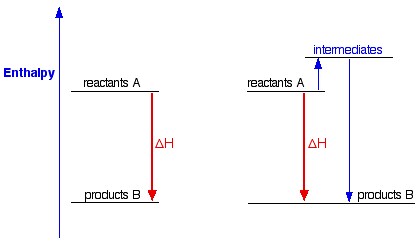
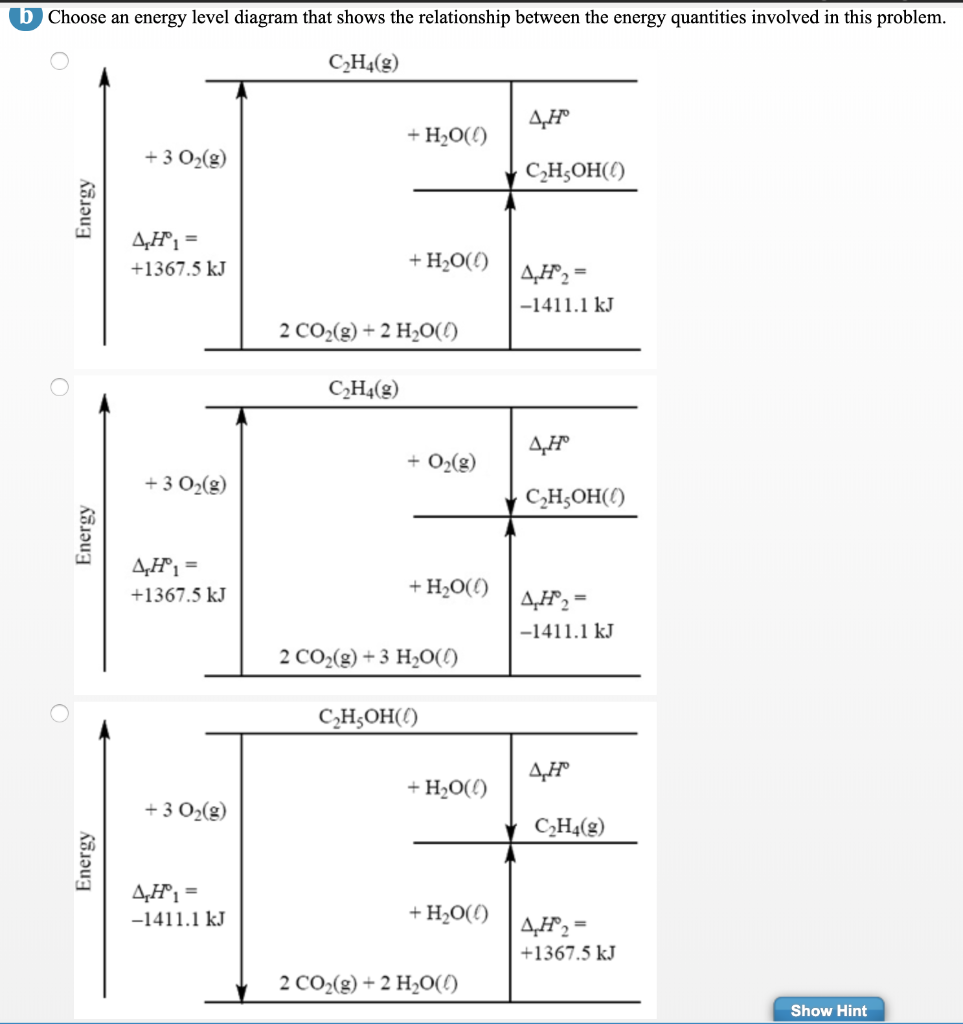
Energetics: 4.32 - Hess' law energy cycles and diagrams The enthalpy level diagram can now be constructed. In this case, the red dotted line is obtained by subtracting the small blue dotted line from the longer blue dotted line. That is, the enthalpy of 4 - 2 = -463 - (-124) = -339 kJ Therefore the standard enthalpy of formation, ΔH f, of phosphorus (III) chloride = -339 kJ top PDF 3.12 Hess's Law - Resources for A-level and GCSE Chemistry Hess's law states that total enthalpy change for a reaction is independent of the route by which the chemical change takes place Hess's law is a version of the first law of thermodynamics, which is that energy is always conserved. 2H (g) + 2Cl(g) H2 + Cl2 2HCl (g) a b ΔH On an energy level diagram the directions of the arrows can Reaction profiles - Exothermic and ... - BBC Bitesize An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction 2. Hess Cycles and Enthalpy Level Diagrams (2) - YouTube Visit for notes on this topic. Thanks for watching!A basic explanation of how Hess' law allows you to calculate the e...
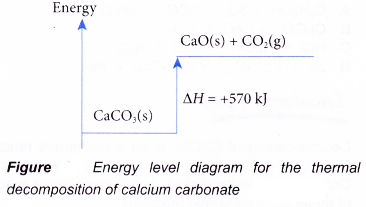
PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... a) Draw an enthalpy level diagram for a neutralization reaction. i) Indicate on your diagram the enthalpy change of the reaction and deduce its sign. ii) Compare the relative stabilities and strengths of the bonds of the reactants and products. [4] iii) Define the term standard enthalpy change of a reaction. [1] 13. IB Chemistry (Ellesmere College) - 5.4 Past Paper Questions C. The enthalpy of the products is less than that of the reactants. D. The reaction is spontaneous at low temperatures but becomes non-spontaneous at high temperatures. 31. According to the enthalpy level diagram below, what is the sign for ∆ H and what term is used to refer to the reaction? Enthalpy of Decomposition of Sodium Hydrogen Carbonate ... Hey guys, Im hoping for help with an assignment I have.We conducted a lab experiment to find the enthalpy of decomposition for sodium hydrogencarbonate using Hesss law.I have a question that asks Why did you find the enthalpy change indirectly?. Well.. Im really not sure.. As far as I know sodium hydrogen carbonate is baking soda, and we regularly heat it to the level it needs to decompose in ... Enthalpy - Chemical energy - Higher Chemistry Revision ... The enthalpy change takes the form of heat given out or absorbed. The heat energy given out or taken in by one mole of a substance can be measure in either joules per mole (J mol -1 ) or more ...
Introducing Enthalpy Change and Enthalpy level diagrams ... Know that enthalpy is the heat change measured at a constant pressure and state the standard conditions Be able to define what we mean by standard enthalpy of a reaction Be able to construct and interpret enthalpy level diagrams showing an energy change Lesson includes discussion points and questions to check understanding.
Enthalpy Level Diagram - aqua ammonia enthalpy ... Enthalpy Level Diagram. Here are a number of highest rated Enthalpy Level Diagram pictures upon internet. We identified it from reliable source. Its submitted by dealing out in the best field. We admit this nice of Enthalpy Level Diagram graphic could possibly be the most trending subject as soon as we portion it in google plus or facebook.
Thermodynamics Enthalpy Entropy Mollier and Steam Tables I… Enthalpy: Enthalpy is defined as the total heat content or total useful energy of a substance. The symbol for enthalpy is “h.” Enthalpy is also considered to be the sum of internal energy “u” and flow energy (or flow work) p.V. This definition of enthalpy can be expressed, mathematically, as follows: h = u + p.V Eq. 1.1 Where,
chemcollective.org › simsChemCollective: Simulations Energy and Enthalpy; Engine Simulation . This simulation allows students to interact with different components of a complex real world phenomenon.The simulation shown is of a 4-stroke internal combustion engine. The thermodynamic cycle being simulated…
Hess's Law and enthalpy change calculations Enthalpy change is simply a particular measure of energy change. You will remember that the enthalpy change is the heat evolved or absorbed during a reaction happening at constant pressure. I have labelled the vertical scale on this particular diagram as enthalpy rather than energy, because we are specifically thinking about enthalpy changes.
enthalpy level diagrams - A level chemistry - The Student Room Rep gems come when your posts are rated by other community members. I take edexcel chemistry a level and have never fully understood how to construct enthalpy level diagrams. I have attached a question about making one - anyone who gets them can you please tell me how you go about constructing one because I find it really confusing.
PDF Measurement of an enthalpy change - chemrevise Step 3 : calculate H the enthalpy change per mole which might be called the enthalpy change of neutralisation H = Q/ no of moles = 2821.5/0.05 = 56430 J mol-1 = -56.4 kJ mol-1 to 3 sf Exothermic and so is given a minus sign Remember in these questions: sign, unit, Note the mass equals the mass of acid + the mass of alkali, as they are both ...
en.wikipedia.org › wiki › InsulinInsulin - Wikipedia The blood insulin level can be measured in international units, such as µIU/mL or in molar concentration, such as pmol/L, where 1 µIU/mL equals 6.945 pmol/L. A typical blood level between meals is 8–11 μIU/mL (57–79 pmol/L). Signal transduction
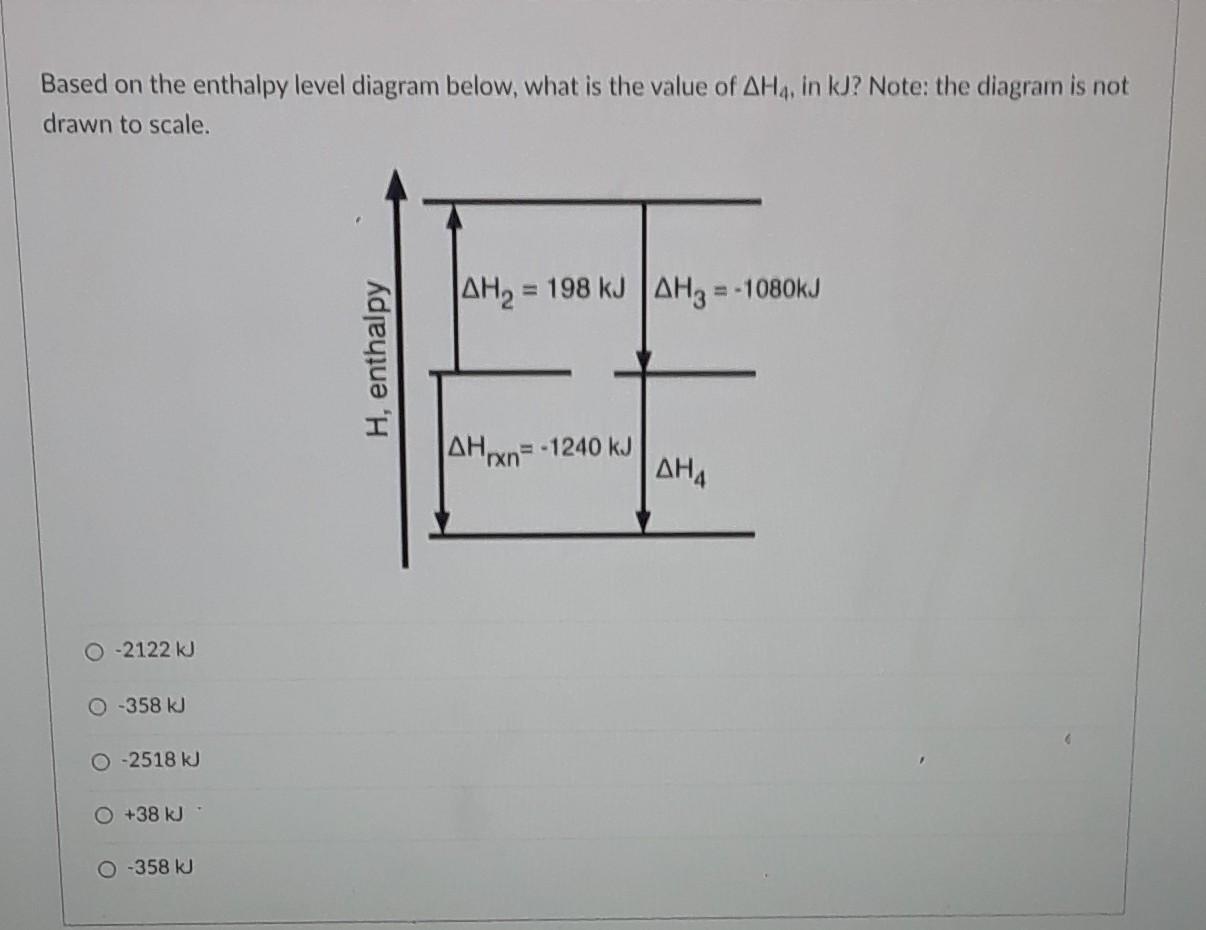
23 Consider the following enthalpy level diagram What is ... 23. Consider the following enthalpy level diagram. What is the standard enthalpy change of the following reaction? N 2 (g) + 2O 2 (g) → 2NO 2 (g) A. − 292.0 kJ mol − B. − 68.0 kJ mol − C. +68.0 kJ mol − 1 D. +292.0 kJ mol − 1 24.
Solved Draw the enthalpy level diagram for and calculate ... Draw the enthalpy level diagram for and calculate the enthalpy of solution for CaSO 4 given that its enthalpy of hydrations is -2608 kJ/mol and its lattice energy is -2591 kJ/mol.
Enthalpy Level Diagrams (2.1.1) | Edexcel International A ... Enthalpy Level Diagrams The total chemical energy inside a substance is called the enthalpy (or heat content) When chemical reactions take place, changes in chemical energy take place and therefore the enthalpy changes An enthalpy change is represented by the symbol Δ H (Δ= change; H = enthalpy) An enthalpy change can be positive or negative
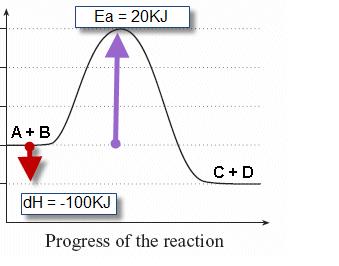
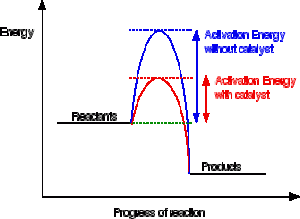
energetics MC quiz Flashcards | Quizlet Which of the quantities in the enthalpy level diagram below is (are) affected by the use of a catalyst? Time A. I only B. III only C. I and II only D. II and III only c Consider the following equations. Mg (s) + O2 (g) → MgO (s) ∆H ο = -602 kJ H2 (g) + O2 (g) → H2O (g) ∆H ο = -242 kJ What is the ∆H° value (in kJ) for the following reaction?
Quiz & Worksheet - Drawing & Labeling Enthalpy Diagrams ... Which of the following enthalpy diagrams best depicts the following reaction: Glucose + Oxygen goes to carbon dioxide and water. Delta H = -298 kJ/mol and activation energy = 70 kJ/mol. Create ...
qualifications.pearson.com › content › damCore practical 8: Calculate the enthalpy change for the ... 3. Reaction 1 – diagram shows exothermic reaction Reaction 2 – diagram shows endothermic reaction Reaction 3 – diagram shows endothermic reaction 4. Polystyrene is a better insulator than glass. Therefore, less heat energy is lost to/gained from the surroundings, so temperature changes are more accurate.
3.12 Hesses law - Resources for A-level and GCSE Chemistry 03.04.2015 · Hess’s law states that total enthalpy change for a reaction is independent of the route by which the chemical change takes place Hess’s law is a version of the first law of thermodynamics, which is that energy is always conserved. 2H (g) + 2Cl(g) H2 + Cl 2 2HCl (g) a b ΔH On an energy level diagram the directions of the arrows can
How to Draw & Label Enthalpy Diagrams - Video & Lesson ... A complete enthalpy diagram will include starting energy, ending energy, and E a and delta H. This enthalpy diagram has starting products, ending products, delta H, and activation energy labeled...
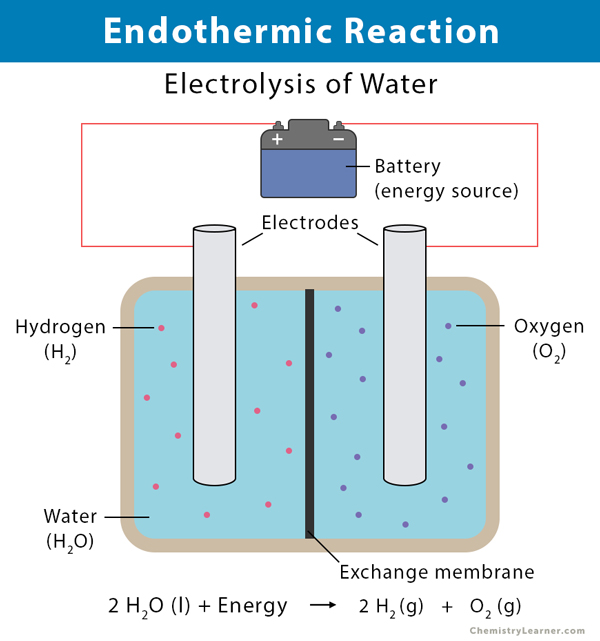
What is an enthalpy diagram? | Socratic An enthalpy diagram shows the change in enthalpy of the reaction as the chemicals move from reactants to products. The change in enthalpy is a fancy term for the change in thermal energy of the system at constant pressure. Typically we classify reactions as either endothermic or endothermic.
Enthalpy Level Diagrams (1.8.2) | Edexcel A Level ... Enthalpy Level Diagrams The total chemical energy inside a substance is called the enthalpy (or heat content) When chemical reactions take place, changes in chemical energy take place and therefore the enthalpy changes An enthalpy change is represented by the symbol Δ H (Δ= change; H = enthalpy) An enthalpy change can be positive or negative
PDF Exothermic enthalpy diagram - Tangente Mag Gives or takes in heat energy, enthalpy change occurs.ΔH = Hproducts - HreactantsWhen petrol is burned enthalpy change is what really matters.ENTHALPY LEVEL DIAGRAMS:For Exothermic reaction ΔH is negative, it means the system loses energy to the surroundings. The products formed will have less energy than the reactants.
















Comments
Post a Comment